Transgenic Plants
Definition, Examples, Applications and Advantages
Definition
The term transgenic plants refers to the plants whose DNA is modified through genetic engineering. This means that one or several genes from a different species are introduced and combined with the genetic material of the plant which changes the traits of the original genome.
While horizontal gene transfer has been shown to occur naturally in nature (between plants that grow close to each other), various artificial techniques are used to insert gene sequences to some plants with the aim of increasing yields, making them more tolerant of various environmental conditions, or making them more resistant to given biotic stresses, etc.
For this reason, transgenic plants are particularly valuable in agriculture as well as in various industries (e.g. in the pharmaceutical industry).
* The first transgenic plant was produced in 1982. It was a tobacco plant that exhibited resistance to antibiotics.
* The new genetic material inserted into the genome of a plant can be derived from a different plant or from a different species.
Examples
Since 1983, genetic engineering has continued to be used on different types of plants to produce desired traits. In particular, these techniques have been used to improve certain traits of corn, tomato, banana, soybean, and tobacco among other plants and crops.
While there are many examples of transgenic plants, there are three main techniques used to introduce gene sequences into plant cells.
These include:
Vector-mediated gene transfer (also known as indirect gene transfer or vector-mediated transformation)
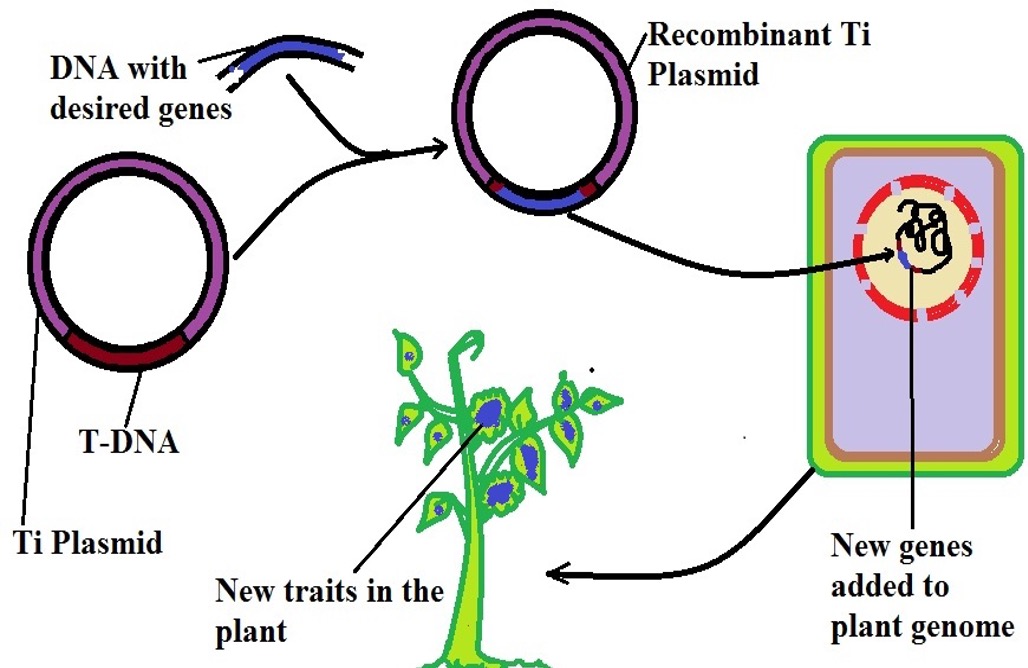
As the name suggests, this technique involves the use of vectors to transport specific genes into the target cell so that they can undergo replication and be expressed. This allows the plant to exhibit the desired characteristics.
While some viruses are used for this technique, two members of the genus Agrobacterium are commonly preferred for this transformation. This is largely due to the fact that they have proven effective in a wide range of plants. In particular, this technique is used in various monocots including maize wheat, and barley among others.
Under normal circumstances, the bacterium Agrobacterium tumefaciens (which is naturally occurring in soil) can sense a wound on the plant surface and introduce some of its genes into the plant cell causing crown gall diseases.
Through the same mechanism, the bacterium Agrobacterium is responsible for hairy root disease in various plants. When the plant is wounded, it releases certain molecules that are detected by the bacteria through a protein known as VirA (located on the bacterial cell surface).
Once they are recognized, the protein activates another protein known as VirG in the cell which in turn activates Vir genes in the bacterial plasmid. This activation triggers the genes to produce endonucleases (VirD1 and VirD2) which cleave at the 25 base pairs on the plasmid to release a single strand of T-DNA. Attached to the endonuclease VirD2, this strand is transported into the plant cell where it's integrated into the genome of the plant.
Although expression of genes in the strand results in the production of several products, some of the most important products include auxin and cytokinin which causes the plant cell to divide rapidly.
As a result, a crown gall tumor develops. In addition to the two hormones, expression of the genes also results in the production of opines which provide carbon and nitrogen required by the bacteria for survival.
Taking advantage of this mechanism, scientists first obtain the T-DNA and introduce it into a plasmid that can be easily altered. This is important given that it allows for a short DNA sequence that borders the transcription unit to be replaced with the genes of interest. The genes of interest are then introduced to the plant cells through infection of the plant with the bacteria.
These genes are integrated into the plant genome and expressed following replication. Given that genes of interest had been introduced into the plasmid, expression of the genes does not produce negative outcomes such as a tumor or opines.
The following is a diagrammatic representation of vector-mediated gene transfer:
Particle-mediated transformation (particle bombardment or gene gun method)
Unlike vector-mediated transformation where the gene of interest is first inserted into a plasmid before being introduced into the plant cell, particle-mediated transformation involves directly inserting genetic material with genes of interest into the cell so that it can be integrated into the genome of the plant.
For this method, a number of metal particles can be used for gene gun bombardment. These include rhodium, gold, and tungsten among others. During this technique, the genetic material (DNA) is first precipitated on to the metal microparticles (microparticles may range from 0.45 to 1.5um in diameter).
In this case, the metal particles act as microcarriers given that they carry the genetic material to be introduced into the plant cell. These carriers are then coated inside plastic tubing. The tube is then cut into short tubes (which act as cartridges) of about half an inch in length using a tube cutter. If the tubes are not used immediately, then they are stored at 4 degrees C.
Here, it's important to ensure that they are kept in a tightly closed vial that contains a desiccant to keep the vial dry. When ready to use, the cartridges are inserted into a cartridge holder which can hold about 12 cartridges. The cartridge holder is then inserted into the gene gun and secured in place.
When the trigger button is pressed, a helium gas pulse is then used to push the microcarriers so that they can bombard plant cells. This helium gas pressure provides enough force that allows the microparticles/microcarriers to punch holes through the cell wall and introduce the genetic material into the cells.
In the cell, some of the particles may be localized in the cytoplasm while others are localized in the nucleus. Regardless, the genetic material separates from the carriers which allows it to combine with the DNA of the plant cell and produce desired characteristics.
* Given that gene guns are used to directly insert new genetic material with desired genes into plant cells using force, the technique can be used for different types of plants, both monocots, and dicots.
For instance, it has been used to transform the genetic material of:
- Soybeans
- Maize/corn
- Barley
- Onions
- Rice
- Melon
* Unlike vector-mediated gene transfer, particle-mediated transformation is easy to perform and has been shown to be very efficient. In addition, it can be used to introduce new genes into different types of plant cells within a short period of time. This makes it one of the most efficient methods of gene transfer.
a- Gas pressure moves down the barrel
b- Pressure raptures the disc
c- The macrocarrier with the DNA coated particles is stopped by stopping screen
d- DNA coated particles reach the plant cells
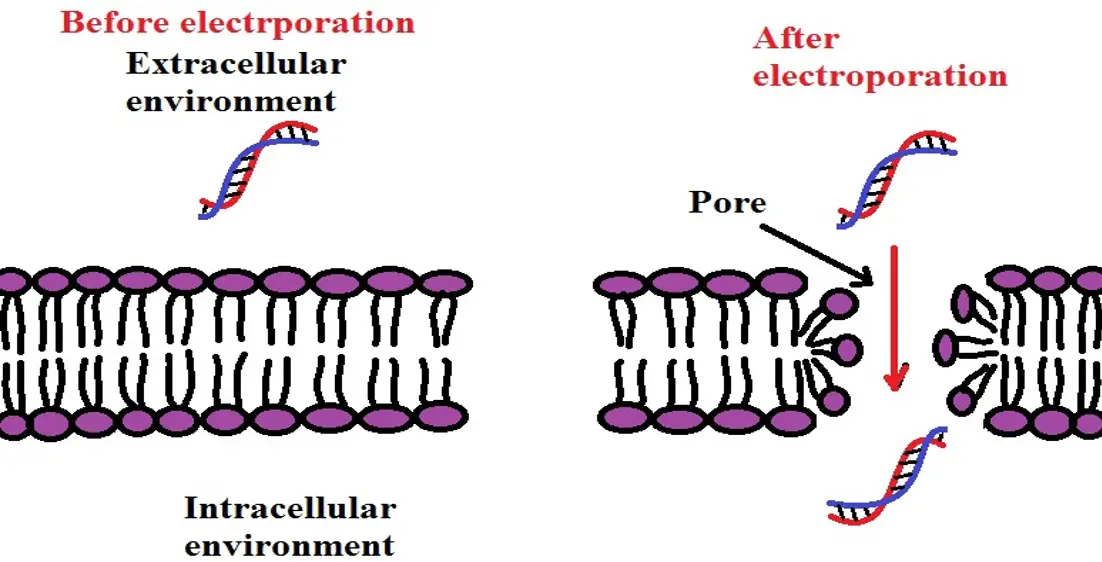
Direct DNA absorption (gene transfer through electroporation)
Like particle-mediated transformation, direct DNA absorption through electroporation is also a direct method of gene transfer. This is because it involves directly inserting new genetic information into the plant cell without using a mediator (vector). Here, the primary goal is to create pores on the cell surface (also known as electropores) through which genetic material can enter the cell.
For this method, the plant cells to be used are first incubated in a buffer that contains the foreign DNA. This is commonly known as a DNA bath and serves to prepare the cells for electroporation. Here, the temperature range and period of incubation is dependent on the type of plant cells as well as the type of foreign DNA being introduced into these cells.
Following the incubation period, the buffer is subjected to electrical impulses that create temporary pores on the cell membrane of the plant cell. Given that these cells are bathed in a buffer that contained the foreign DNA, this DNA can then easily enter the cell through these pores before they close.
As the method is generally used for protoplasts, some researchers first use chemical treatments to create pores on the surface (cell wall) of plant cells before subjecting the cells to electroporation.
Regardless, this technique is rarely used to transform plants due to the challenges presented by the cell wall.
Applications: Applications of transgenic plants
With regards to transgenic plants, there are two main areas of applications.
These include:
In Agriculture
As these techniques involve changing the genomic information of plants, the agricultural sector is one of the main areas of agriculture.
Since 1982, the technology has been used to transform the characteristics of different types of plants ranging from corn and barley to onion and tomato.
Here, gene transfer is used for various functions ranging from increasing yields (crops and animals feeds etc) as well as increasing plant/crop tolerance to various biological and environmental factors.
Biopharming/Pharmaceutical Industry
Apart from the agricultural sector, gene transfer in plants has also found many applications in the pharmaceutical industry. Gene transfer has been used for the large scale production of various proteins and chemicals that plants would not naturally produce.
As compared to some of the other cells used in biopharmaceuticals, transgenic plants are suggested to be more cost-effective. Moreover, they have in place the machinery that can transform molecules into given structures that can serve the required biological activities.
Role of Transgenic Plants as Bioreactors or Biofactories
As is the case with many other cells, plant cells have biological machinery involved in various processes. Here, the information (blueprint) required for these processes is contained in the genetic material.
By introducing new information in the form of new genes, it has become possible for researchers to take advantage of these processes to produce various proteins including vaccines and antibodies among others.
While bacteria and other types of cells have been used as bioreactors, transgenic plants have been gaining more attention in recent years because they are less expensive and are capable of post-translational modifications involved in the production of complex proteins.
Advantages of Transgenic Plants
As mentioned, the methods and technologies used to produce transgenic plants are applied in several industries/sectors. This is because transgenic plants present many advantages compared to natural ones.
Some of the main benefits of transgenic plants include:
They are resistant to various biotic and abiotic stress - Biotic stresses include those that result from other organisms in nature (bacteria, viruses, fungi, etc) while abiotic stresses are the type of stresses that result from the environmental conditions in which given plants are grown.
By introducing specific genes, researchers have managed to make many types of plants/crops resistant to these stressors. In the process, farmers are able to avoid the heavy costs associated with plant/crop damage resulting from biotic and abiotic stressors.
Increased yields - The other advantage of transgenic plants is that they give high yields compared to natural plants. In general, there are two main reasons as to why these plants give higher yields. The first reason is that it has become possible for researchers to introduce genes that can transform plants resulting in higher yields compared to natural plants/crops.
As well, by introducing genes that allow the plant to withstand biotic and abiotic stressors, the plant is able to survive various environmental conditions that would otherwise cause significant damage to the plants.
High quality of yields - One of the biggest advantages of transgenic plants is that they produce higher quality yields. As mentioned, there are many stressors in nature that can affect plant growth and yields. In addition, various chemicals used for the purposes of controlling insects, pests, and weeds, etc have also been shown to affect sensitive plants.
Through gene transfer, researchers have been able to introduce new characteristics into different types of plants allowing them to withstand these stressors. As a result, these plants provide high-quality yields because they are not significantly affected by factors that affect other plants and their yields.
* While there have been environmental and health concerns from the public, there is currently no substantial evidence that these plants can be harmful.
Return to understanding Micropropagation
Return to learning about Plant Biology
Return to Differences between Animal Cells and Plant Cells
Return to Bacteria - Size, Shape and Arrangement
Return from Transgenic Plants to MicroscopeMaster home
References
Ibrahim Ilker Ozyigit. (2020). Gene transfer to plants by electroporation: methods and applications.
Parvaiz Ahmada, Muhammad Ashraf, Muhammad Younis, Xiangyang Hud, Ashwani Kumare,
Nudrat Aisha Akram and Al-Qurainy. (2011). Role of transgenic plants in agriculture and biopharming.
Phillips, T. (2008) Genetically modified organisms (GMOs): Transgenic crops and recombinant DNA technology. Nature Education 1(1):213
Rivera AL, Gómez-Lim, Fernández. and Loske AM. (2014). Genetic Transformation of Cells using Physical Methods.
Links
Find out how to advertise on MicroscopeMaster!