Toxoplasma
Definition, Gondii, Life Cycle, Morphology and Treatment
Definition
First discovered in small desert rodents (gundi in Northern Africa) in 1908, Toxoplasma is a genus of the phylum Apicomplexa and consists of a single species known as Toxoplasma gondii. Because of its ability to infect any warm-blooded animal, the protozoan is ubiquitous in nature making it one of the most widespread parasites across the globe.
Usually the infection (toxoplasmosis) is subclinical in healthy individuals. However, mild to severe cases have been observed among pregnant women and immunocompromised patients (e.g. patients with HIV/AIDs). While only a single species of the genus Toxoplasma has been discovered so far, studies have been able to identify a few strains of the parasite.
Some of the main modes of its transmission include:
- Consumption of undercooked, contaminated meat
- Fecal-oral transfer of the oocysts
- Vertical transmission of the parasite from mother to fetus - transplacental transfer
* The name "Toxoplasma" is derived from a Greek word referring to a crescent shape (bow-shaped).
Classification of the Genus Toxoplasma
· Kingdom - Protista - Generally, the kingdom Protista consists of eukaryotic organism (single-celled or in a colony) other than animal, fungus, or plants. They either exist in the environment as free-living organisms or as parasites or symbionts in multicellular organisms
· Subkingdom - Protozoa - unicellular eukaryotes that either exist as parasites or as free-living organisms
· Phylum: Apicomplexa - consists of intracellular parasites that are characterized by a polarized cell structure, a complex cytoskeletal as well as organellar arrangement.
· Subclass - Coccidiasina - is a subclass of the class Sporozoasida. It's composed of single-celled, spore-forming obligate intracellular parasites
· Order - Eucoccidiorida - consists of spore-forming parasites of the class Conoidasida
· Family - Sarcocystidae - is a family of Apicomplexa that has been associated with many diseases in human beings
· Genus - Toxoplasma
· Species - Toxoplasma gondii
Life Cycle and Characteristics
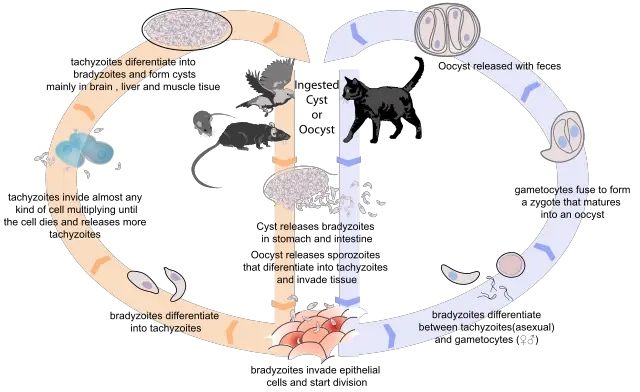
As is the case with a number of other parasites, the life cycle of Toxoplasma gondii is dependent on definitive and intermediate hosts. For Toxoplasma, specifically, felids (domestic and wild cats) serve as definitive hosts while a wide variety of domestic and wild animals (including birds) serve as intermediate hosts.
Because of the unusually wide spectrum of animals (warm-blooded animals) that serve as intermediate hosts, Toxoplasma gondii has been shown to be one of the most common parasites in animals and human beings across the world.
Although only a single species in the genus (Toxoplasma) has been identified so far, the different strains of the species have been associated with varying pathogenicity of the parasite in different types of hosts.
As members of the subclass Coccidiasina, toxoplasma gondii have a complicated life cycle that is characterized by sexual and asexual reproduction.
Sexual Reproduction
As compared to asexual reproduction, sexual reproduction of Toxoplasma gondii occurs within the definitive host. This cycle can be said to start when the host (wild or domestic cat) ingests oocysts/tissue infected with bradyzoite cysts. Once they are ingested, the cyst wall is dissolved by proteolytic enzymes in the stomach and small intestine to release bradyzoites.
The free bradyzoites then penetrate epithelial cells lining the small intestine where they proliferate to form new generations that can undergo sexual and asexual cycles.
In cats, multiplication of bradyzoites within epithelial cells lining the small intestine is known as entero-epithelial cycle. This results in the production of schizonts which give rise to merozoites to form the male and female gametes. Using their flagella, the male gametes swim to the female gametes for fertilization to take place.
Following fertilization of the female gametes, a wall starts to form around the oocysts. Following the rapture of epithelial cells (in which the oocysts develop) the oocysts are released to the environment along with feces thus contributing to asexual reproduction when the oocysts are ingested by an intermediate host.
Asexual Reproduction
Whereas sexual reproduction cycle only involves the definitive host (wild/domestic host), asexual reproduction requires both the definitive and intermediate hosts. To describe this type of reproduction, we can assume that it starts with the release of the oocysts into the environment.
Typically, the oocysts released into the environment along with fecal matter (by the definitive host) tend to be non-infective. At this stage, the oocysts, known as sporonts, are single cells that are environmentally resistant.
Within a period of between 1 to 5 days, the oocysts (sporonts) undergo sporulation to form infective sporocysts. Here, cell development results in the formation of two sporocysts that contain four infective sporozoites each.
* In the environment, the number of days it takes for sporulation to occur is largely dependent on environmental conditions.
* In the environment, sporulated oocysts can survive for a long period of time until they are ingested by the intermediate host.
Once they are ingested, by human beings or any warm-blooded animal (in soil, water, undercooked foods etc), the sporocyst wall is dissolved by proteolytic enzymes thus releasing the sporozoites.
These sporozoites then penetrate epithelial cells lining the small intestine where they undergo endodyogeny (a form of asexual reproduction) to form tachyzoites. The newly formed tachyzoites then spread and actively penetrate other cells of the intermediate host where they are surrounded by a parasitophorous vacuole protecting them from the hosts' immune system.
In this environment, tachyzoites divide asexually to form two progeny within the cells. As they continue dividing, Tachzoites ultimately cause the cells they reside in to break thus releasing as many as 32 tachyzoites that go onto infect new cells. However, this attracts the attention of the immune system which ultimately slows down tachyzoite multiplication.
In response, the tachyzoites penetrate new cells where they are surrounded by a thin wall to form tissue cysts. At this stage, the tachyzoites are commonly referred to as bradyzoites. The tissues formed are common in a number of body tissues and organs including the eyes, cardiac muscle, neural tissue, and various visceral organs where they can last for the hosts’ entire lifetime.
In the event that wild or domestic cats ingest these cysts (by consuming infected birds or other animals) the cyst wall is broken down by proteolytic enzymes to release bradyzoites that penetrate and proliferate within the epithelial cells lining the small intestines.
Attachment and Invasion
Before penetrating the cell, the parasite first has to attach to the surface of the host cell. In order to do this, the parasite relies on receptor-ligand interactions to overcome the repulsive force caused by the net negative charge of both the parasite and host cell membrane.
As compared to many other parasites, Toxoplasma gondii are capable of attaching and penetrating many different types of cells. This has been suggested to be as a result of the parasite being capable of expressing multiple receptors for different types of ligands or a few receptors capable of binding to ligands that are common to a wide variety of cells. One of the proteins that have been associated with this attachment is SAG1 (a parasite antigen).
To invade the host cell, actin/myosin motors within the parasite as well as a number of transmembrane proteins linked to the motors are involved. As compared to phagocytosis, where phagocytes engulf invading organisms and infected cells, penetration of Toxoplasma gondii into cells is rapid with the body of the parasite constricting as it penetrates the cell. Moreover, rearrangement of the cytoskeleton of mammalian cells is not observed during parasite (Toxoplasma gondii) penetration to the cells of the host.
Characteristics and Adaptations
As mentioned in the life cycle of Toxoplasma gondii, there are three main infectious stages of the parasite. These stages have a number of morphological stages that allow them to survive and multiply in the cells of the host.
Tachyzoites
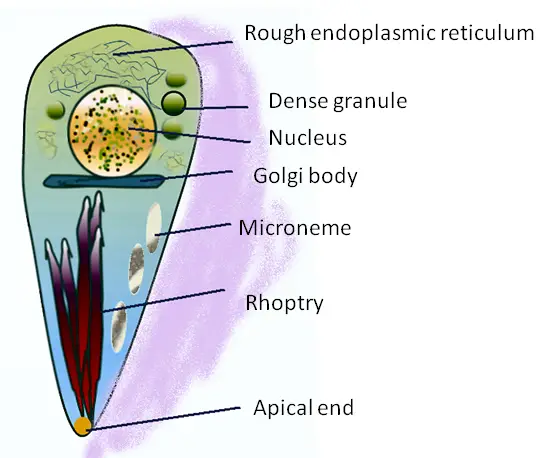
Measuring about 2um in width and 6um in length, tachyzoites often multiply in various cells of the host. They are characterized by a pointed anterior region and rounded posterior giving them a somewhat comma-shaped appearance or crescent-shaped.
They also contain a number of organelles that include apical rings, pellicle, polar rings, rhoptries, micropore, and a nucleus among several other organelles found in eukaryotic cells (E.R, ribosome, mitochondria etc).
Although they lack such specialized structures as flagella and cilia used for locomotion, tachyzoites can glide, extend, retract and rotate as they search for host cells.
Using micropores, micronemes, and conoid among a few other structures, tachyzoites are able to actively penetrate the cells where they then create an environment in which they can survive and proliferate.
According to studies, the conoid tends to tilt, extend, and rotate etc as the parasite interacts with the plasma membrane of the host cells. Using these movements, the parasites are able to actively penetrate the cell. However, in some cases, this is achieved through phagocytosis.
Once they successfully penetrate the cell of the intermediate host, tachyzoites are surrounded by a parasitophorous vacuole followed by the development of a tubulovesicular membranous network within the vacuole. Whereas the vacuole (parasitophorous vacuole) is derived from both the parasite and the cell of the host, the network of tubulovesicular membrane is produced at the posterior end of the parasite (tachyzoite).
In the cells of the intermediate host, tachyzoites divide asexually through a process known as endodyogeny. Here, two progeny produced within the parasite develop and grow until they come into contact with the surface of the parent parasite.
While the inner membrane of the parent parasite gradually disappears, the outer membrane ultimately becomes the plasmalemma of the progeny cells. The cells continue dividing until the cell of the intermediate host ruptures releasing the parasite to search and penetrate new cells.
* Tachyzoites represent the rapidly growing stage of the parasite.
* Generation time of tachyzoites in cells is between 6 and 8 hours.
* Between 64 and 128 parasites accumulate the cell before it raptures.
Bradyzoites
Also known as cystozoites, bradyzoites multiply within the tissue host. Compared to tachyzoites, bradyzoites have been shown to multiply at a slow rate. With regards to morphology, this stage of the parasite is characterized by a spherical/ovoid cell surrounded by a thin cyst wall. However, they may also appear more slender in shape. Generally, their nucleus is located at the posterior end (compared to the nucleus of tachyzoites that is centrally positioned).
Like tachyzoites, bradyzoites also multiply through endodyogeny. Given the parasite at this stage divide at a slower rate, new tissue cysts are small in size, measuring about 5um in diameter. This is because they contain very few bradyzoites (about2).
Over time, the cyst continues to increase in size and the bradyzoites continue to grow and multiply. As a result, older cysts may contain more than 100 bradyzoites and measure about 70um in diameter. However, for the most part, it's the tissue cysts in muscular tissue (intramuscular cysts), rather than those in the brain, that grow to over 70um in size. If left intact, these cysts do not typically cause any harm.
* Compared to tachyzoites, bradyzoites are less susceptible to proteolytic enzymes.
* Over time, the cyst wall becomes thickened enough to resist pressure and tearing.
Cysts containing infectious bradyzoites are known as the essential resting stages. As such, they are spread and cause an infection when ingested by a different host.
Apart from being less susceptible to proteolytic enzymes, bradyzoites have also been shown to accumulate a variety of glycogen in their cytoplasm that serves as a source of energy. Using the glycoprotein (amylopectin) they gain the energy required to invade new cells when need be.
Sporozoites
At the ultrastructure level, sporozoites of Toxoplasma gondii have many similarities to tachyzoites. Compared to tachyzoites, however, sporozoites contain a high abundance of granules in the micronemes, rhopties, and amylopectin. With regards to size, sporozoites are also similar to tachyzoites, measuring about 2um in width and 8um in length.
Some of the cell organelles that can be identified in sporozoites include:
- Golgi complex
- Subterminal nucleus
- Centrioles
- Apicoplast
- Microneme
- Endoplasmic reticulum
In the environment, sporozoites within the oocysts are protected from various harsh environmental conditions (desiccation, UV etc) by the oocyst/sporocyst wall. They have also been shown to overcome these stressors by relying on some of their own defenses. Following ingestion, this wall is degraded allowing the sporozoites to be released.
Disease Mechanism
For the most part, toxoplasmosis (the infection caused by Toxoplasma gondii) remains asymptomatic in healthy human beings. This is largely due to the fact that the body’s' immune system is able to control the parasite thus preventing the infection from getting out of control.
The infection may vary from mild to severe in some cases. For instance, while anyone can be infected by the parasite, pathogenicity is largely dependent on virulence of the stain as well as host susceptibility. In animals, this has been demonstrated through research studies where adults infected with the parasite exhibited no symptoms while young rats died.
In human beings, the infection can be acquired congenitally or postnatally. Typically, congenital infections occur when women are infected during pregnancy. Postnatal infections, on the other hand, occur as a result of a number of events including contact with felid feces (when ingested), organ transplant from an infected individual or blood transfusion from an infected person.
When women are infected during pregnancy, the parasite has been shown to cause a number of pregnancy outcomes and diseases including stillbirth, birth defects (e.g. hydrocephalus), and abortion, etc.
Depending on the individual and the sight of infection, some of the following symptoms maybe exhibited:
- Headache
- Muscle pain
- Rashes
- Nausea
- Eye pain - in the case of retinochoroiditis
- Abdominal pain
- Sore throat
- Fever
- Focal lesion in the placenta
- Convulsions
As previously mentioned, the rapidly dividing tachyzoites induce inflammation which activates an immune response. This is particularly important for the parasite in two major ways. First, the parasites invade and replicate within these cells and increase in number. Secondly, they are transported to various tissues across the body (brain, muscle, etc) where they infect new cells.
While some of the tachyzoites are destroyed by the responding immune cells, those that survive are transported to various tissues where they give rise to bradyzoites that form cysts in these tissues. For patients with a compromised immune system, the parasite can continue infecting new cells without any restriction. This can cause significant damage to cells, ultimately affecting the normal functioning of organs which can prove fatal.
Reactivation of cysts among those with compromised immune systems has been associated with such conditions as Toxoplasmic encephalitis which can result in epileptic seizures, etc.
Prevention
Toxoplasmosis can be prevented through a number of hygienic practices. These include washing hands after handling raw meat and cooking meat to about 170 degrees Celsius. Women are advised to avoid contact with cats or at least avoiding cat litter boxes in order to prevent congenital infections. Through these practices, it becomes possible to prevent or at least minimize infections among the vulnerable.
Treatment
Treatment of the Toxoplasmosis is typically recommended in the event of visceral disease in cases where symptoms become persistent and severe. There are also treatment options for pregnant women with acute infections, neonates and patients with compromised immune systems.
For patients with a compromised immune system and persistent symptoms, the following treatment options are recommended:
- Pyrimethamine
- Sulfadiazine
- Leucovorin
Chronic maintenance therapy (used to prevent relapses) include:
- Atovaquone
- Atovaquone, and sulfadiazine
- Clindamycin, pyrimethamine, and leucovorin
- Atovaquone, pyrimethamine, and leucovorin
Return to Parasites under the Microscope and Parasitology
Return from Toxoplasma to MicroscopeMaster home
References
Frenkel J.K. (2000) Biology of Toxoplasma gondii. In: Ambroise-Thomas P., Petersen P.E. (eds) Congenital toxoplasmosis. Springer.
J. P. Dubey, D. S. Lindsay, and C. A. Speer. (1998). Structures of Toxoplasma gondii Tachyzoites, Bradyzoites, and Sporozoites and Biology and Development of Tissue Cysts. nbci.
L. M. Randall and C. A. Hunter. (2011). Parasite dissemination and the pathogenesis of toxoplasmosis. nbci.
Michael W. Black and John C. Boothroyd. (2000). Lytic Cycle of Toxoplasma gondii. Department of Microbiology and Immunology, Stanford University School of Medicine,
Stanford, California.
Stephanie J. Melchor and Sarah E. Ewald. (2019). Disease Tolerance in Toxoplasma Infection. Front. Cell. Infect. Microbiol., 06 June 2019.
Links
https://pubs.usgs.gov/circ/1389/pdf/circ1389_highres.pdf
Find out how to advertise on MicroscopeMaster!