Senescence
Definition, Markers, Cancer, Vs Apoptosis
Definition
Essentially, senescence may be described as the state in which cells irreversibly stop dividing. While this does not mean cell death, cells that enter senescence can no longer divide to produce daughter cells. While this is often caused by telomere shortening, it may also be a result of a number of factors including unrepaired DNA damage and other cell stresses affecting the DNA.
However, it's worth noting that for the most part this state is beneficial in that it prevents some of the negative outcomes (e.g. development of malignant cells) that may otherwise take place if cell division is allowed to continue among such cells.
Role of Telomeres in Normal Cell Division and Senescence
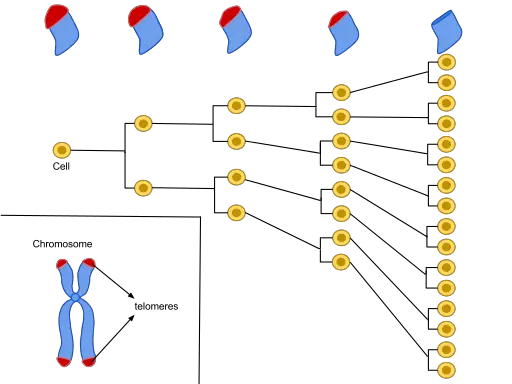
Under normal circumstances, various mitotic cells (e.g. epithelial cells of the skin, fibroblasts, endothelial cells, etc) undergo mitosis which allows for growth and tissue regeneration (where dead and dying cells are replaced). During mitotic division, the DNA also replicates thus allowing each newly formed daughter cell to retain a copy of the original DNA of the parent cell.
Located at both ends of the DNA strands are protective caps known as telomeres (consisting of repetitive nucleotide sequences). While they contain repetitive DNA structures, telomeres cannot code for any protein given that they do not have genes required for this process.
Telomeres are also protected by a coating known as shelterin that serves to prevent the fusion of chromosome ends. With each cycle of cell division, the telomere becomes shorter given that the DNA polymerase system does not copy the entire length of the DNA strand.
According to studies, cells can undergo 60 to 70 cycles before the telomere becomes excessively short putting the DNA strand at risk of damage.
At this point, the DNA damage response (cellular pathways that monitor integrity and respond to DNA damage) is activated which prevents further cell division. At this point, the cells can no longer divide this being a permanent state of growth arrest.
* A healthy human cell can only divide a given number of times (60-70 times) before it enters the senescent state. This limit is known as the Hayflick limit.
* In embryonic tissues, telomerase continues adding DNA to both ends of the chromosomes thereby providing a template for DNA synthesis. This is an important process that prevents telomere erosion among these cells.
Because adult cells lack this ribonucleoprotein complex (telomerase), the telomeres and shelterin are progressively eroded ultimately resulting in senescence.
* In human beings, telomere erosion has been associated with aging deterioration.
While telomere shortening is the main cause of senescence, some of the other factors that can contribute to this process include:
- DNA damage - (e.g. broken DNA double-strand)
- Abnormal protein expression
- Damage or changes to DNA-associated proteins - e.g. damaged chromatin
- Metabolic dysfunctions
- Oxidative stress - Reactive oxygen species produced through metabolism
- Damage to mitochondrial DNA
Markers and Pathways Regulating Senescence
With over 107 proteins identified as possible senescence markers, some of the proteins that have been validated thus far include PLD3, DEP1, STX4, ARMX3, and EBP50. Both in vitro (in culture) and in vivo (in body tissues), a combination of these proteins have proved beneficial when it comes to identifying senescent cells.
Apart from these markers, some of the other well studied markers of senescence include p53 (TP53) and p16/Rb (which are also tumor suppressor pathways).
p53 pathway - Following DNA damage, high amounts of γH2Ax and 53BP1 are deposited in chromatin resulting in kinase cascades that ultimately activate p53 (a gene responsible for protein-coding to regulate cell cycle).
In turn, this gene induces the transcription of cyclin-dependent kinase inhibitor p21CIP1 thus blocking activities of CDK4/6. This results in hypophosphorylated retinoblastoma and consequently causes the cell to exit cell cycle.
* Like p53 pathways, p16 mediated senescence also activates senescence through the retinoblastoma pathway. Here, the action of cyclin-dependent kinases is inhibited which ultimately results in G1 cell cycle arrest.
Types of Senescence
Based on the kinetics involved in senescent process, two main types of senescence have been identified.
These include:
· Acute senescence - Also known as transient senescence, acute senescence is common in such biological processes as wound healing and tissue repair. For instance, following wound healing, myofibroblasts have been shown to undergo senescence.
Here, acute senescence prevents further production of fibroblasts and myofibroblasts and their activities in preventing/minimizing fibrosis. Ultimately, these cells are eliminated following the activation of immune clearance cells.
· Chronic senescence - As compared to acute senescence, chronic senescence (persistent senescence) is influenced by persistent cellular stressors of gradual macromolecular damage.
This type of senescence is also characterized by high SASP (senescence-associated secretory phenotype) heterogeneity and tends to have damaging effects in cells and tissues. On the other hand, chronic senescence has been associated with age-related diseases among the elderly.
Characteristics and Benefits
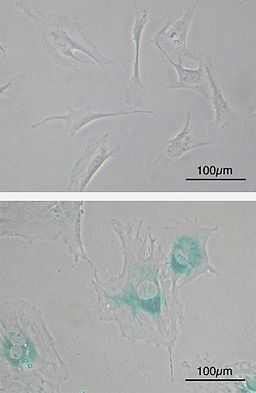
As compared to the other mitotic cells in the body of a healthy individual, senescent cells can no longer divide. Because they also start expressing other types of genes than they did before, these cells also start to look different and thus have different morphological characteristics.
For instance, compared to other cells, senescent cells have a characteristic flattened and enlarged morphology. They also exhibit a number of new molecular markers including SAHF (senescence-associated heterochromatin foci) and lipofuscin granules in addition to releasing SASP factors.
While they are not readily eliminated from the body, senescent cells are unable to continue dividing like normal cells which is a big advantage among healthy individuals. This is largely because of the fact that by entering the senescent state, damage to the DNA is prevented.
Here, it's worth keeping in mind that normally, it's the telomeres that undergo erosion during each cycle of cell division. Therefore, when the cell enters the senescent state, the DNA strand is protected from damage.
On the other hand, in a case where the DNA had been damaged (thus activating the senescence pathways), this prevents damaged DNA from being replicated.
Then, senescence processes prevent the formation of new daughter cells that would otherwise inherit abnormalities of the parent cells. This has also been shown to prevent the proliferation of cancer cells associated with damaged cell DNA.
The downside to this, however, is that because the cells can no longer divide, tissue repair is affected. There have also been cases where senescent cells have been associated with such age-related diseases as cataracts.
* In the body of an adult, some of the cells (referred to as post-mitotic cells e.g. neurons and cells of the heart muscle) do not replicate and thus do not normally experience replicative senescence. However, they can be induced to undergo senescence in some cases.
Difference between Senescence and Apoptosis
While both senescence and apoptosis ultimately contribute to cell death, the two are essentially different in several ways. For instance, whereas senescence promotes the cell aging and deterioration (while preventing division of these cells), apoptosis is responsible for cell death.
While senescence may occur as a result of DNA damage, it is normally activated when telomeres are depleted over time. To protect DNA from being damaged following telomere erosion, senescence permanently arrests cell cycle at the G1 phase.
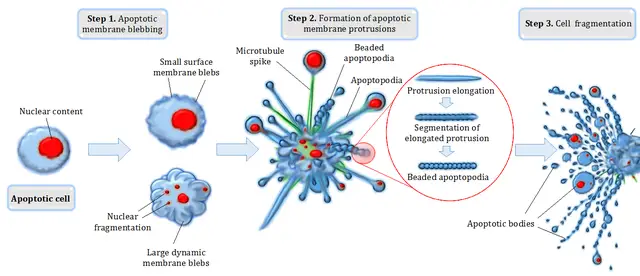
Here, then, the cell is allowed to continue living, but it cannot divide to produce new cells. This continues until the cell completely deteriorates and is eliminated by other cells. Apoptosis, on the other hand, is a programmed cell death process that may be caused by various pathological and physiological factors.
In the event that the cell is damaged by invading micro-organisms or simply nears the end of its lifespan (e.g. red blood cells), a tightly regulated intracellular proteolytic cascade contribute to the death of the cell.
Here, the cell is actively involved in its death (where it shrinks and ultimately fragments). Whereas permanent arrest of cell division is the main characteristic during senescence, chromosome condensation and ultimately cell shrinking and fragmentation are the main characteristics of apoptosis.
Return from Senescence to MicroscopeMaster home
References
Amancio Carnero. (2013). Markers of Cellular Senescence. ResearchGate.
Bennett G Childs, Darren J Baker, James L Kirkland, Judith Campisi, and Jan M van Deursen. (2014). Senescence and apoptosis: dueling or complementary cell fates? ncbi.
Domhnall McHugh and Jesús Gil. (2018). Senescence and aging: Causes, consequences, and therapeutic avenues.
M Althubiti, et al. (2014). Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death and Disease.
Slavica Dodig, Ivana Čepelak, and Ivan Pavić. (2019). Hallmarks of senescence and aging. ncbi.
Links
https://www.researchgate.net/publication/10840030_Defining_senescence_and_death
https://www.nature.com/subjects/senescence
Find out how to advertise on MicroscopeMaster!