Pseudomonas Aeruginosa
Gram Stain, Culture Characteristics, Infection
Antibiotic Resistance and Antimicrobial Susceptibility Testing
Water Treatment
Introduction
Pseudomonas aeruginosa is a member of the genus Pseudomonas. They are Gram-negative bacteria commonly found in various moist environments.
While the bacterium is a pathogen that is responsible for various hospital-acquired infections, these infections are particularly severe among individuals with a compromised immune system.
Culture Characteristics
As mentioned, Pseudomonas aeruginosa is ubiquitous in nature and can be found in various moist environments. This has been attributed to the fact that the bacterium is characterized by extensive metabolic diversity allowing it to live and thrive in various ecological niches.
With regards to microbial culture, this is particularly important given that a variety of growth media can be used. While the bacterium grows well at 37 degrees C, it can also survive at the temperature range between 4 and 42 degrees C.
To study various characteristics of Pseudomonas aeruginosa, some of the media used include Pseudomonas isolation agar, LB Broth, King A, and MOPS ([3-(N-morpholino) propane-sulfonic acid]).
To grow the bacteria in Pseudomonas Isolation Agar, the plates (clean and sterile plates) are first allowed to warm at room temperature. The agar is then poured into the plates and allowed to dry before inoculation. Once the agar has dried, the specimen has to be inoculated within the shortest time possible after it has been collected.
Generally, inoculation involves streaking the specimen over the surface of the agar (covering about a third of the surface). Here, streaking must be done using a sterile loop in order to avoid contaminating the medium with other microorganisms.
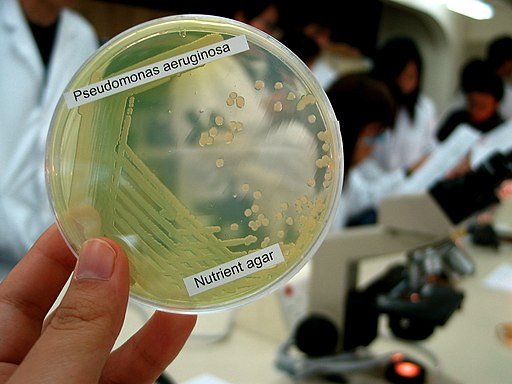
Following inoculation, the plate or plates are incubated for one (1) day at 37 degrees C aerobically. After 24, Pseudomonas aeruginosa appear as blue-green colonies. If other Pseudomonas or non-fermenting bacteria are present, they are not blue-green in color.
It's worth noting that the pigments (water-soluble pigments) produced by Pseudomonas aeruginosa may vary depending on the medium or strain of bacteria.
Some of the most common pigments include; pyocyanin which is blue-green in color, pyoverdine which is yellow-green in color, and pyorubin which is red-brown in color.
Apart from the pigmented colonies, Pseudomonas aeruginosa grown in media is also characterized by grape-like smell. Being a non-fermentor, the bacterium is also associated with acid production in culture rather than gases which are commonly associated with fermenting bacteria.
Studies have shown that in the presence of nitrate, Pseudomonas aeruginosa can grow slowly in an anaerobic environment at about 42 degrees C.
Apart from the media mentioned above, Pseudomonas aeruginosa can also be grown in MacConkey agar (a bacterial culture medium commonly used to grow lactose fermenting bacteria). While the bacterium cannot use lactose present in this medium, it survives on peptone.
In MacConkey agar, Pseudomonas aeruginosa forms flat and smooth colonies that are between 2 and 3mm in diameter. Generally, these colonies have regular margins and have an alligator skin-like appearance when viewed from above.
Some of the other characteristics of Pseudomonas aeruginosa in culture include:
· Colorless in MacConkey agar - This is attributed to the fact that the bacteria does not ferment lactose
· In Centrimide agar - Pseudomonas aeruginosa colonies (greenish-blue in color) are medium-sized and characterized by an irregular growth
· In Nutrient agar - Pseudomonas aeruginosa are associated with several odors ranging from a sweet to earth smell
Gram Stain
Growing bacteria in culture is important in that it allows researchers to analyze and study various characteristics (smell, texture, and shape of colonies, the color of the colony, etc) of the organism.
Using Gram staining technique makes it possible for researchers to not only identify morphological characteristics of bacterial cells, but also differentiate them based on their cell wall components.
Requirements for Gram stain include:
Sample - Pseudomonas aeruginosa grown in culture can be used.
- Glass slides
- Gram stain reagents
- Wire loop
- Water
- Burner
Procedure
· If the sample is obtained from a culture plate, then it's necessary to add a drop of water onto the glass slide before aseptically adding a small amount of the bacteria. This can be achieved by using a sterile wire loop to place the sample on to the drop of water
· Using the wire loop or glass stir stick/rod, make a good smear of the sample at the middle of the glass slide
· Allow the slide to air dry - If bacteria from the broth was used, then it's important to allow all the liquid to evaporate before fixing
· Once the slide has dried, heat fix by passing over the flame - You can pass the slide over the flame about three times and avoid overheating
· Flood the slide with crystal violet for about 1 minute and then rinse with water
· Treat the slide with Gram's Iodine for about 1 minute and then gently rinse with water
· Add a few drops of alcohol (95 percent alcohol) on to the slide and then rinse with water
· Flood the slide with Safranin (counterstain) and then rinse with water
· Add a drop of immersion oil onto the slide and observe under the microscope
* Preparing several slides (about 3 slides) is always recommended
Observation
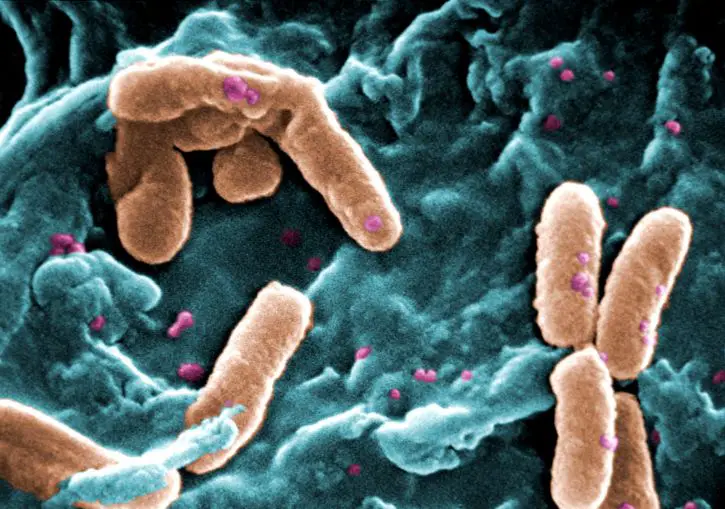
When viewed under the microscope, Pseudomonas aeruginosa will appear as reddish/pink rods. This indicates that they are Gram-negative bacteria given that they are unable to retain the primary stain (crystal violet).
Under high magnification, studies have shown Pseudomonas aeruginosa to range from 0.5 to 0.8um in diameter and 1.5 to 3.0um in length (rod-like bacteria). They are also characterized by a single polar flagellum used for motility.
For some of the strains, studies have revealed the presence of two to three polar flagella used for movement. In addition to the flagella, Pseudomonas aeruginosa also possess pili on their surface which are used for adhesion and a form of motility known as twitching motility.
Read more about Gram positive and Gram negative bacteria.
Infection
As mentioned, Pseudomonas aeruginosa is responsible for various hospital-acquired infections.
According to a report that was published by the European Center for Disease Prevention and Control in 2012, about 9 percent of all health-care-associated infections are caused by Pseudomonas aeruginosa making it the fourth most common pathogen responsible for infections in European hospitals.
For the most part, the bacterium is an opportunistic pathogen of the mucosal tissues. However, infections of the cornea (the eye) and the urinary tract have been reported.
Depending on the patient, infections of the respiratory tract range from nosocomial pneumonia to lung infections among patients with cystic fibrosis.
Nosocomial Infections
Essentially, nosocomial infections are hospital-acquired infections and thus occur post-admission.
Some of these infections include:
Burn wound infections - In addition to Staphylococcus aureus and Streptococcus pyrogens, Pseudomonas aeruginosa is one of the leading causes of invasive infections among burn patients. Here, the site of injury (from the burn) allows for the successive invasion of the bacterium.
Apart from entering the body through the injured skin, the bacterium has been shown to gain entry as a result of inhalation injury thus increasing the risk of respiratory infections.
Bacteremia - Pseudomonas aeruginosa is also one of the main causes of nosocomial bacteremia. Given that this particular organism has been shown to be resistant to various antimicrobials, these infections have been shown to result in higher mortality as compared to some of the other pathogens responsible for bacteremia.
Hospital and ventilator-associated pneumonia - Given that the respiratory tract provides favorable conditions for life, the bacterium is easily able to cause chronic and acute infections among patients with cystic fibrosis.
In addition, the pathogen has also been shown to be one of the main causes of ventilator-associated pneumonia (VAP) particularly in the case of increased duration of mechanical ventilation.
Urinary Tract Infection
Pseudomonas aeruginosa has been shown to be particularly effective at forming surface-associated biofilms. For patients who use catheters, the bacterium has been shown to form biofilm on the surface of these catheters (indwelling catheters) and ultimately cause an infection as they proliferate.
Pathogenesis and Virulence Factors of Pseudomonas aeruginosa
Pathogenesis of Pseudomonas aeruginosa is made possible by several virulence factors that include:
Lipopolysaccharide - Lipopolysaccharide is one of the main components of the outer membrane of Pseudomonas aeruginosa. In addition to Lipid A, a hydrophobic domain, this component of the outer membrane also consists of O-antigen (distal polysaccharide) which not only determines the serotype of the organism but also activates the immune system of the host.
Eventually, the polysaccharide results in dysregulated inflammation which has been associated with morbidity and mortality.
Flagellum - As mentioned, Pseudomonas aeruginosa contains a single polar flagellum used for swimming in moist environments. In addition to motility, this structure has also been shown to play an important role in attachment to the epithelium, invasion as well as biofilm formation.
Type IV Pili - Type IV pili located on the surface of Pseudomonas aeruginosa play an important role in adhesion to various cells thus promoting infections. In addition to adhesion, the pili have also been shown to be involved in twitching motility which in turn promotes the formation of biofilms.
Some of the other factors that promote the pathogenesis of Pseudomonas aeruginosa include:
· Exotoxin A - associated with local tissue damage and gradual invasion
· Proteases - Pseudomonas aeruginosa produces a number of proteases including LasB and alkaline protease that destroy tissue
· Alginate - is one of the main components of mucoid exopolysaccharide capsule and plays an important role in cell adherence
Antibiotic Resistance
Following an infection, Pseudomonas aeruginosa has been shown to be resistant to a variety of antimicrobials.
There are several modes of resistance which include:
Intrinsic resistance to antibiotics - Essentially, intrinsic antibiotic resistance refers to the innate ability of bacteria to evade the impacts of antibiotics. This may be achieved through various structural and functional characteristics.
Some of the mechanisms through which Pseudomonas aeruginosa is able to diminish the efficacy of various antibiotics (intrinsically) include:
Permeability of the outer membrane - In Pseudomonas aeruginosa, the outer membrane is an asymmetric bilayer that consists of phospholipid and LPS (Lipopolysaccharides). It also consists of porins that are responsible for the beta-barrel protein channels.
The composition of this membrane makes it very restrictive and is responsible for limiting the penetration of antibiotics. However, the membrane does not completely prevent this penetration. Rather, slow uptake of these molecules contributes to intrinsic resistance.
Efflux systems - Apart from the limiting outer membrane, Pseudomonas aeruginosa is also able to pump out toxic compounds. In particular, studies have shown proteins associated with the resistance-nodulation-division (RND) family to be largely involved in this activity in this bacterium.
Here, the proteins make up cytoplasmic membrane transporters and outer membrane porin channel proteins involved in expelling toxic compounds out of the cell. In cases where these pumps are overexpressed, the bacterium gradually develops resistance to a variety of drugs.
Antibiotic-inactivating enzymes - One of the other factors that contribute to antibiotic resistance is the ability of the bacterium to produce enzymes capable of breaking down and modifying antibiotics.
In particular, Pseudomonas aeruginosa has been shown to produce such enzymes as hydrolytic enzyme β-lactamase which breaks the amide bond of certain antibiotics. In doing so, the drug is rendered ineffective against the pathogen.
Also: How do antibiotics kill bacteria?
Acquired Antibiotic Resistance
Acquired antibiotic resistance is the second mechanism through which Pseudomonas aeruginosa have developed antibiotic resistance.
This is achieved through:
Mutational change - Mutational change is particularly beneficial for the pathogen as modification of antibiotic targets allows them to evade the intended actions of the drug. This may involve the overexpression of efflux pumps and thus the ability of the bacterium to remove toxic substances from the cell.
Acquired resistance genes - Bacteria have been shown to be capable of acquiring genes through horizontal transfer. In the case of various P. aeruginosa strains, the acquisition of resistance genes allows the bacterium to develop resistance to various antibiotics. This transfer may occur through conjugation, transduction, or transformation.
Adaptive Antibiotic Resistance
The last mechanism of resistance against antibiotics is through adaptive antibiotic resistance. Generally, this is achieved through the formation of a biofilm. A biofilm refers to adhesion or clustering of microorganisms on a given surface.
In the host, the biofilm formed by Pseudomonas aeruginosa is then covered by a matrix. As compared to other pathogenic cells, these cells tend to be less sensitive to antimicrobial agents.
Antimicrobial Susceptibility Testing
As mentioned, Pseudomonas aeruginosa has been shown to be resistant to a number of antibiotics. This is due to a number of virulence factors associated with the organism. For this reason, Antimicrobial Susceptibility Testing is an important test to determine the most effective treatment to treat infections caused by the bacterium.
Essentially, Antimicrobial Susceptibility Testing involves placing a microorganism in contact with antibiotics in order to study whether or not the organism will grow in the presence of the antibiotics being used.
Here, Mueller-Hinton agar using disc diffusion can be used for the test given that the technique is applicable for a wide range of non fastidious bacteria with little change of error.
Agar preparation involves the following steps:
· 38 grams of the medium is suspended in a liter of purified water and mixed
· The mixture is then heated for about 1 minute with frequent agitation in order to ensure that the contents mix properly
· The agar is autoclaved for 15 minutes at 121 degrees C and then allowed to cool to 45 degrees C
· The agar is then poured into Petri dishes to a depth of about 4mm
· The plates are allowed to solidify at room temperature and investigated to ensure that the pH remains 7.3 +11 at 25 degrees C
* Once the researcher is ready to test sensitivity/susceptibility of the pathogen the bacterium is inoculated and the antimicrobial disks placed on the culture (using sterile forceps). The plates are then inverted and incubated at 37 degrees C for between 16 to 18 hours.
* Antibiotic disks contain the drug being tested.
Some of the antibiotics used to test sensitivity/susceptibility of Pseudomonas aeruginosa include:
- Ticarcillin
- Aztreonam
- Ciprofloxacin
- Kanamycin
- Cefepime
Water Treatment
While Pseudomonas aeruginosa is ubiquitous in nature, it's commonly found in moist environments. For this reason, it's also found in various water bodies including lakes and rivers, etc. In order to prevent possible infections, water treatment is necessary.
Antimicrobial susceptibility testing is used to determine the most effective water disinfectant. Here, this test also considers the impact that the disinfectants being tested can have on the health of those who use the water.
Based on previous studies, Pseudomonas aeruginosa has been shown to be susceptible to several disinfectants including ozone, iodine, chloramines, and chlorine. For this reason, they are commonly used to treat water in many developed and developing nations.
While UV (ultraviolet) disinfection has proven effective for a number of other microorganisms in water, this form of treatment has been shown to be less effective when it comes to Pseudomonas aeruginosa. As a result, the aforementioned water treatment options are often recommended.
See also: Pseudomonas syringae, Pseudomonas fluorescens
Return to Pseudomonas main page
Return to learning about Proteobacteria
Return to Bacteria under a microscope main page
Return to more on Bacteria - Size, Shape and Arrangement
Return from Pseudomonas aeruginosa to MicroscopeMaster home
References
Barbara H. Iglewski. (1996). Pseudomonas. Medical Microbiology. 4th edition.
Benie CKD et al. (2017). Prevalence and Antibiotic Resistance of
Pseudomonas aeruginosa Isolated from Bovine Meat, Fresh Fish and Smoked Fish.
Kristina D Mena and Charles P Gerba. (2009). Risk assessment of Pseudomonas aeruginosa in water.
Patricia Ruiz-Garbajosa and Rafael Cantón. (2017). Epidemiology of antibiotic resistance in Pseudomonas aeruginosa. Implications for empiric and definitive therapy.
Robert B. Fick, Jr. (1993). Pseudomonas Aeruginosa the Opportunist.
Links
https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/PseudomonasIsolationAgar.htm
Find out how to advertise on MicroscopeMaster!