Platelets
Function, Thrombocytopenia, Aggregation, Count/Microscopy
Overview
Also known as thrombocytes, platelets are blood cells that are primarily involved in hemostasis. As such, they are involved in wound healing which in turn stops bleeding.
Apart from their role in hemostasis, platelets are also involved in angiogenesis and innate immunity. Like the red blood cells, they are anucleate with a characteristic discoid shape. In the body, the normal platelet count ranges from 150,000 to about 400,000 cells per microliter of blood. An abnormally high or low count of these cells is indicative of various health conditions.
A very low count (thrombocytopenia) indicates that the bone marrow is making abnormally few platelets or that the platelets are being destroyed at a high rate which can have serious health complications.
Thrombopoiesis (Platelet Production)
* In mammals, thrombocytes are known as platelets.
As with all blood cells, the production of platelets starts with the differentiation of multipotent hematopoietic stem cells (hemocytoblast) located in the red bone marrow. This differentiation results in the production of myeloid stem cells that differentiates to produce a number of cells (red blood cells, granulocytes, and platelets).
In the presence of thrombopoietin, a megakaryocyte growth and development factor produced in the liver and kidneys, the myeloid stem cell is influenced to produce megakaryoblast which differentiates into a promegakaryocyte. In turn, this gives rise to megakaryocytes that ultimately produce platelets.
Here, it's worth noting that megakaryocytes are large cells that can average 75um in diameter (10 to 15 times larger than a normal blood cell). As such, they have to undergo fragmentation in order to pass through the sinusoidal capillaries and get into the bloodstream. It's these fragments that form the platelets (cytoplasmic fragments).
Platelet Characteristics
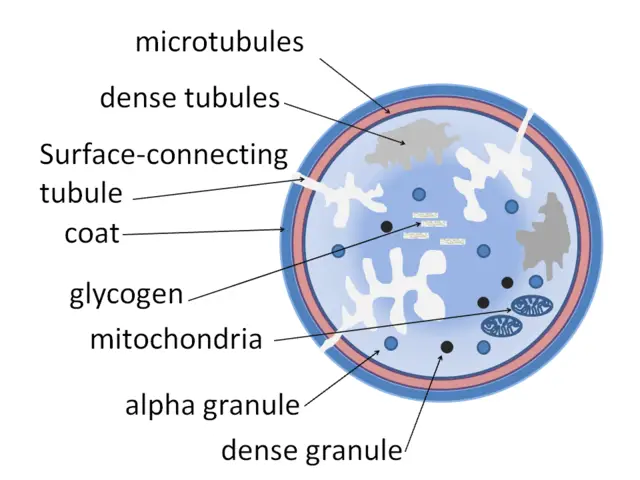
- Small in size ranging between 2 and 3um in diameter- This is about 20 percent the diameter of a normal blood cell
- Lifespan of about 10 days
- About 100 billion new platelets are produced on a daily basis to maintain the normal platelet count
- Each megakaryocyte produces about 1,000 platelets
- Contain granules involved in clotting to stop bleeding
- Are the lightest blood cells
- Lack a nucleus and have a general disc shape
- Contain a number of organelles including mitochondria, peroxisomes and lysosomes
Platelet Functions
Hemostasis
Hemostasis refers to the process that stops hemorrhage from damaged blood vessels thus preventing excess blood loss. It's the primary function of platelets that can be divided into three main phases/processes that include activation, adhesion, and aggregation.
Under normal circumstances, the endothelial wall (consisting of endothelial cells that make up the inner wall of the vessels) is sufficiently smooth which prevents cells (or anything else) from sticking. This is particularly important given that it helps prevent the vessels from blocking. However, when this layer is damaged (the endothelial layer), fibers are exposed to the fluid blood.
* Damage to the blood vessels also result in vasoconstriction (to reduce blood loss) as well as the recruitment of macrophages (following the release of ATP and various inflammatory mediators).
* Normal endothelial cells also secrete nitric oxide and prostacyclin that prevent platelet adhesion.
As a result of injury to the blood vessel, cells in the affected site produce very little nitric oxide and prostacyclin. As a result, platelets are able to come into contact and even adhere to the wall.
Apart from reduced nitric oxide and prostacyclin, platelet adhesion is promoted by protein receptors (glycoprotein receptors) on their surface. These receptors bind to collagen with the help of an adhesive protein known as von Willebrand Factor.
Here, the protein serves to bind other proteins (collagen) at the affected site. This being the first process of hemostasis, it allows for platelets to properly adhere to the affected site which activates them.
Following adhesion, platelets are activated and undergo degranulation. This results in the release of a number of substances including ADP (adenosine diphosphate), platelet activating factors as well as serotonin. In addition to degranulation, the general shape of platelets also changes into a more pseudopodal morphology that allows for better adhesion.
The serotonin released during degranulation contributes to vasoconstriction, which reduces the amount of blood passing through the vessel. This is important given that it reduces the amount of blood lost through the injured vessel. ADP, on the other hand, serves to promote platelet aggregation.
Apart from serotonin and ADP, degranulation also releases calcium that plays an important role in secondary hemostasis which helps stabilize the plug.
The next and last phase/process of hemostasis is known as platelet aggregation. Here, platelet pseudopods are extended which causes the clumping and aggregation of platelets. This results in the formation of the primary platelet plug marking the end of primary hemostasis.
* During secondary hemostasis, fibrin links together on top of the platelet plug to create a mesh that reinforces the platelet plug. Here, the end result is known as a clot.
Angiogenesis
While platelets play an important role in homeostasis, they are also involved in angiogenesis. As such, it not only contributes to tissue repair in wound healing but also promotes the formation of new blood vessels from existing ones.
One of the instances in which platelets have been shown to play a role in angiogenesis is in tumor angiogenesis. Here, it is worth noting that as tumorous cells continue to grow and increase in numbers, there is a need for new blood vessels to supply oxygen and nutrients required for proper cell development/growth.
Platelets may promote tumor angiogenesis through the secretion of a number of pro-angiogenic factors such as MMP9, VEGF-A, and phospholipids among others. On the other hand, they have been shown to achieve this by binding to the endothelial cells. Here, fibrin, a product of platelets, supports the formation of new vessels by activating endothelial cells.
Role of Platelets in Innate Immunity
Because of their ability to detect and rapidly respond to endothelial injuries, platelets play an important role in immunity. For instance, as already mentioned, platelets contain a variety of molecules that are released during degranulation. Some of these molecules are inflammatory as well as bioactive molecules that attract various effector cells to action.
Having adhered to the injured vessels, platelets have been shown to recruit neutrophils, guiding to the affected site through cell-cell interactions. Platelets, along with neutrophils, recruit monocytes to the affected site. Here, platelets promote this action by ensuring endothelial cell integrity.
* Platelets also promote the production of macrophages by influencing the differentiation of monocytes into macrophages thus contributing to appropriate immune reactions.
In addition to recruiting various cells of the immune system, platelets have also been shown to act as effector cells in innate immunity. This is achieved by not only detecting endothelial injury, but also invading pathogens when they invade tissue/blood having penetrated through the affected site.
Collagen is exposed (as well as various membrane protein) which allows for platelet aggregation. This is followed by activation and consequent release of a number of platelet agonists (e.g. thrombin) that stimulate increased platelet recruitment. While this stops blood loss, it also provides defense against further microbial infection.
* Platelets also express chemokine receptors that allow for signal detection in the event of an infection. This makes it possible for platelets to accumulate rapidly when a given site is infected.
Platelets are Antimicrobials
Through their interaction with such microbes as fungi, bacteria, and viruses, platelets also serve anti-microbial functions. This is because platelets express various receptors that allow the cells to quickly identify invading organisms.
Some of the most common examples of these receptors are GP1b, TLRs, and FcγRIIa (these being bacterial receptors). These receptors allow platelets to bind to bacteria and release various anti-microbial substances that ultimately destroy the organism.
For instance, following contact with the invading bacteria, platelets, using bacterial receptors, bind to these organisms and release anti-microbial products known as platelet microbial proteins (PMPs such as defensins and thymosin b4) that act against the bacteria.
Through such actions, platelets are able to protect the body against the following microbes:
- S. aureus
- E. coli
- S. pyogenes
- S. pneumoniae
- Leishmania promastigotes
- Toxoplasma gondii
Platelet Count/Microscopy
A platelet count refers to a test used to determine the number of platelets in a given sample of blood. While it can be used for educational purposes, platelet count is part of a health examination that may be used to diagnose or monitor health conditions associated with platelets.
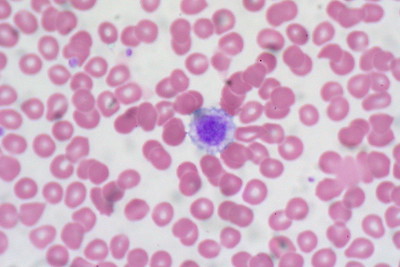
As previously mentioned, the normal range of platelet count in an individual (human being) is between 150,000 and 400,000. By examining a peripheral blood smear, it's possible to approximate the number of platelets in a sample.
Examination of Peripheral Blood Smears
Requirements
- Blood sample - (This may be preserved in an EDTA tube) Ethylene Diamine Tetra Acetic acid
- Leishmania stain
- Glass slides
- Oil immersion
- Compound microscope
Procedure
· If the blood is preserved in an EDTA tube, invert the tube about 10 times in order to mix the cells- allows for equal distribution of cells in the sample
· Using a pipette, place a drop blood (from the tube) about 1/4inch from the frosted part of the slide - may also be achieved by simply pressing the open side of the tube onto the slide and turning it upside down
· Using another clean slide or coverslip at an angle (about 30 degree angle), create a smear/film by dragging the drop of blood backward - should be done rapidly with one stroke in order to create a good smear
· Place the slide on a rack and allow it to air dry
· Stain the smear using Leishman stain and wash away excess stain - Staining may also involve the use of Romanowsky stain
· Allow the smear to air dry and observe the slide under the microscope using oil immersion (at high magnification)
Observation
When viewed under the light microscope, platelets will appear as small refractile bodies spread among red blood cells in an unstained smear. However, they will seem blue or purple in color when stained.
Counting is performed manually in order to estimate the number of platelets in a sample. If the number of platelets falls between 8 and 25, then the sample contains a normal platelet count.
* When counting the number of platelets, examining several fields of view is advised.
Apart from using peripheral blood smears, the number of platelets in a sample may be estimated using a counting chamber or automated hematology analyzers. However, while the former is time-consuming and tedious, using automated hematology analyzers has also been associated with errors resulting from the presence of particles in the sample.
While using peripheral blood smears may not be the most ideal method, it provides relatively reliable results. This is particularly due to the fact that it is possible to examine several fields of view on a slide for more reliable results.
Thrombocytopenia
Through a platelet count test, it's possible to determine (approximate) the number of platelets in a blood sample. A platelet count of less than 150,000 per microliter is known as thrombocytopenia and may affect the ability of the blood to clot. In most cases, however, patients rarely display any symptoms.
It's worth noting that for different patients/individuals, this may vary. For instance, in some patients, platelet counts may be over 50,000 per microliter of blood, but lower than 150,000. In such cases, patients rarely exhibit any symptoms.
In cases where this number falls below 50,000 (30,000 to 50,000), studies have shown the condition to manifest as purpura (characterized by purple-colored spots on the skin).
In cases where the count falls to between 10,000 and 30,000 per microliter of blood, this may result in bleeding and minimal trauma in patients. This number, however, may fall further to between 5,000 and 10,000 cells per microliter which has been associated with spontaneous bleeding.
Depending on the patient, there are three major causes of thrombocytopenia, these include:
· Reduced production - Reduced production of platelets has been associated with a number of factors including viral infections, liver disease as well as vitamin deficiencies among others. Here, any of the factors may interfere with the normal production of the cells from the bone marrow.
· Elevated destruction - thrombocytopenia may occur when platelets are destroyed by drugs, cells of the immune system or idiopathic pregnancy etc. These factors may directly or indirectly influence the destruction of platelet cells in the body thus resulting in low platelet count.
· Sequestration - In biology, sequestration refers to the net removal of a given substance. With regards to thrombocytopenia, this has been shown to occur as a result of spleen enlargement causing it to function abnormally.
Here the spleen may sequester up to 90 percent of the platelets thereby causing a significant drop in the number of platelets in circulation. As well, this has been shown to occur during pregnancy.
Apart from the symptoms mentioned above, some of the other symptoms associated with thrombocytopenia include:
- General fatigue - as a result of excessive blood loss
- Bleeding from nose and gums
- Presence of blood in urine
- Unusually heavy menstrual bleeding in females
- Deep vein thrombosis
Thrombocytosis
A platelet count may also reveal an abnormally high number of platelets in a blood sample, a condition known as thrombocytosis.
Currently, two types of thrombocytosis have been identified:
Primary thrombocytosis - Also known as essential thrombocytosis, primary thrombocytosis occurs when an increased number of platelets is produced by abnormal cells in the bone marrow. The main cause of primary thrombocytosis remains unknown.
Secondary thrombocytosis - Secondary thrombocytosis is also characterized by an abnormally high number of platelets in the body. It may be caused by a number of factors such as an infection, cancer and iron deficiency.
* While many patients may not exhibit any symptoms, thrombocytosis has been associated with such health complications as a heart attack, stroke as well as unusual clotting in the abdomen and blood vessels.
Return from Platelets to MicroscopeMaster home
References
Constanza E. Martínez1, Patricio C. Smith and Verónica A. Palma Alvarado. (2015). The influence of platelet-derived products on angiogenesis and tissue repair: a concise update.
Gauer RL, Braun MM. (2012). Thrombocytopenia. NCBI.
Julie Rayes, Joshua H. Bourne, Alexander Brill, and Steve P. Watson. (2019). The dual role of platelet‐innate immune cell interactions in thrombo‐inflammation. Wiley Online Library.
Lisa Repsold et al. (2017). An overview of the role of platelets in angiogenesis, apoptosis and autophagy in chronic myeloid leukaemia.
Thomas Gremmel, Andrew L. Frelinger III, Alan D. Michelson. (2016). Platelet Physiology.
Umarani MK, Shashidhar H.B. (2007). Estimation of platelet count from peripheral blood smear based on platelet: red blood cell ratio. A prospective study in a tertiary care hospital.
Yangu Zhang. (2016). Platelets – Multifaceted players in tumor progression and vascular function. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1271.
Links
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5096834/
https://www.medicinenet.com/thrombocytopenia_low_platelet_count/article.htm
Find out how to advertise on MicroscopeMaster!