Neural Progenitor Cells (NPCs)
** Function, Markers and Transfection
** Differentiation and Proliferation
Neural Progenitor Cells (NPCs) are a type of progenitor cell that give rise to different types of cells (neuronal and glial cells) in the central nervous system. Although it was initially thought that these cells can only be found in the central nervous system of embryos, new studies showed that they are also present in the central nervous system of newborn babies and the adult mammalian brain.
In the brain of mammalian adults, they are localized within the subgranular zone within the dentate gyrus as well as the subventricular zone which surrounds the lateral ventricles.
* Because of the presence of neural progenitor cells in the adult brain, it's now widely believed that the brain has the potential to repair itself following an injury (neural repair).
Proliferation of Neural Progenitor Cells
Proliferation of cells simply refer to their division which allows them to increase in number. Here, cells first have to grow and then divide. Naturally, however, this process is controlled in order to maintain a given number of cells. Like many other progenitor cells, neural progenitor cells are capable of self-renewal and proliferation.
These processes are both important in that they ensure that a given number of progenitors are always present to give rise to new cells. Therefore, without the proliferation phase, the production of new neural and glial cells would be affected or stopped.
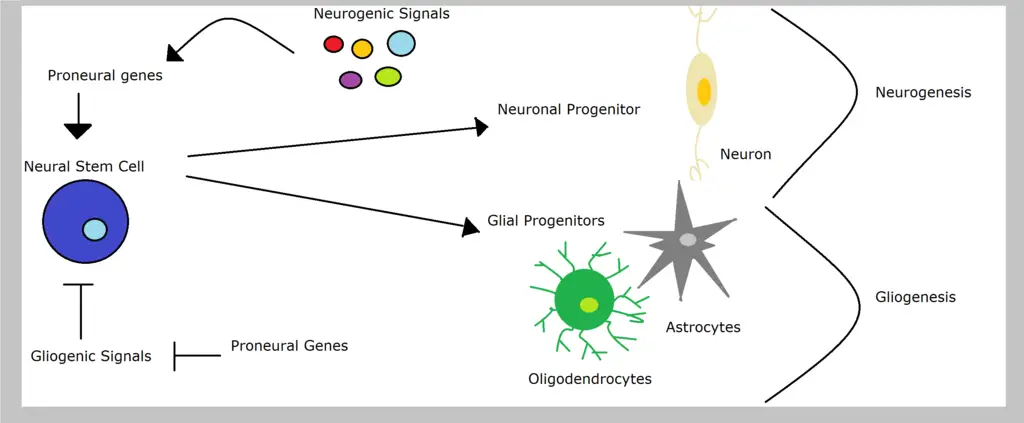
Neural progenitors themselves originate from neural stem cells which divide to give rise to one stem cell (self-renewal) and a progenitor cell (differentiation). This is known as asymmetric neurogenic division.
While there are three types of division for neural progenitor cells (early symmetric, direct and indirect asymmetric), it's through the early symmetric phase that these cells divide and increase in number.
* The primary neural progenitors are known as neuroepithelial progenitors or neuroepithelial cells. These are the cells responsible for producing all the other progenitors in the central nervous system.
As mentioned, proliferation (and division of progenitor cells in general) is highly regulated to ensure that a given number of cells is maintained. With regard to symmetric and asymmetric division of progenitors of the central nervous system, the Lethal giant larvae gene (Lgl) in Drosophila is one of the factors involved in this regulation.
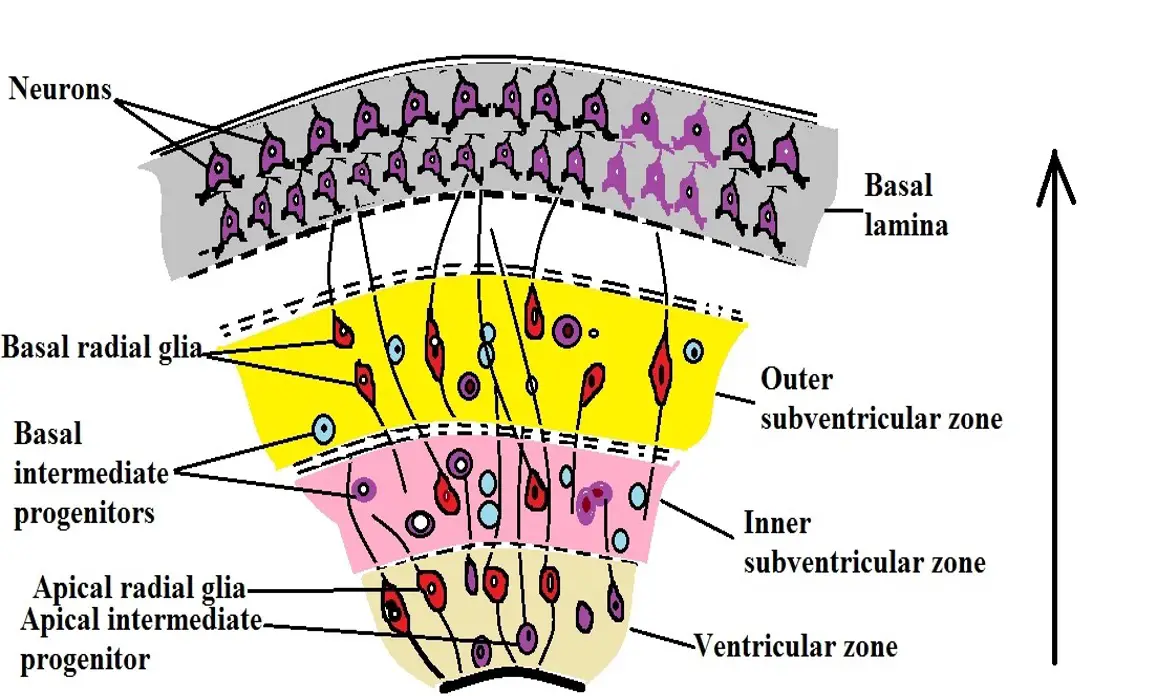
Before and during symmetric division, studies have shown there to be a cleavage plane that is perpendicular to the lumenal surface of the ventricular zone. This is therefore viewed as promoting symmetric division and thus promoting proliferation.
Like other cells, this type of division gives rise to daughter cells that resemble the parent. For this reason, the process contributes to proliferation where more progenitor cells are produced. These progenitor cells can then undergo differentiation to give rise to more differentiated and functional cells.
Some of the characteristics associated with proliferating neural progenitors include:
- Are elongated
- Extended cytoplasmic process
- Have a conspicuous apicobasal polarity
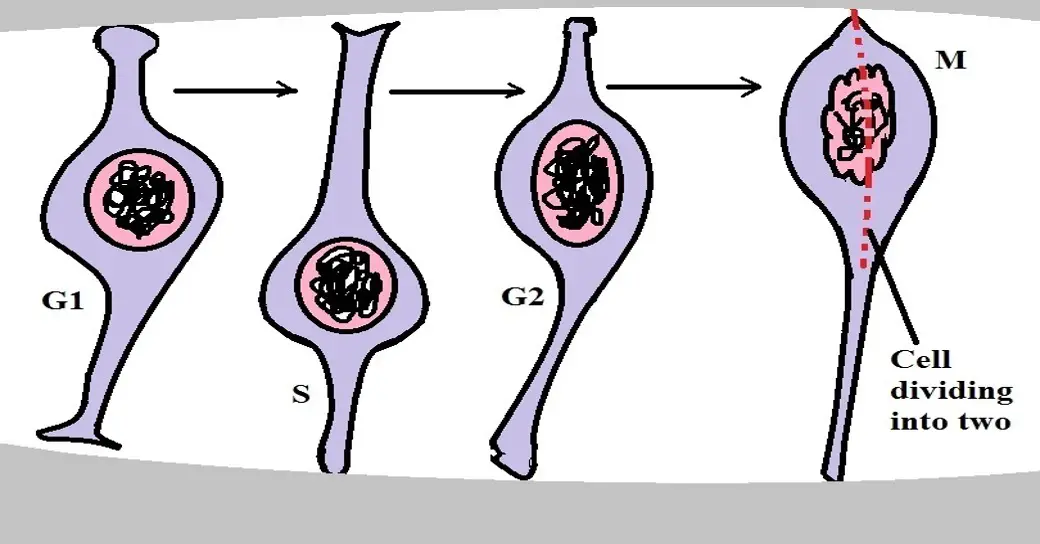
* Proliferation of the neural progenitor involves several phases which include the G1, S, G2, and the M Phase.
* Like neuroepithelial cells, radial glial cells (produced by neuroepithelial cells) are also capable of self-renewal. However, they differentiate to give rise to more functional cells of the nervous system (neurons and glial cells).
Differentiation of Neural Progenitor Cells
Radial glial cells are elongated (more elongated compared to neuroepithelial cells) with a cell body that resides in the ventricular zone within the neural tube. Following the differentiation of these cells, they give rise to a number of cells including neurons, ependymal cells, astrocytes as well as oligodendrocytes.
Whereas proliferation (through symmetric division) results in an increase in the number of progenitors, differentiation of these cells, through asymmetric division, results in the production of one progenitor and one differentiated cell.
As is the case with symmetric division, the axis of polarity, as well as the orientation of the spindle fibers (the cleavage plane), determines the type of division that the cell will undergo.
In symmetric division, the mitotic orientation is perpendicular to the cell's polar axis which results in proliferation. During differentiation, however, studies have shown that the mitotic orientation tends to be parallel to the polar axis of the cell resulting in asymmetric division.
* While neural progenitors have the potential to repair damaged neurons in the central nervous system, studies have shown the intrinsic repair mechanism of the brain to be very ineffective.
Transfection
Transfection refers to the process through which nucleic acids are introduced into eukaryotic cells in order to study the functions of given genes.
Some of the methods used in transfection include:
- Viral methods
- Chemical or lipid based-methods - e.g. using molecules like polymers
- Electroporation
- Particle-based methods
For established cells or labs that have been manipulated in the laboratory for this specific purpose, there are various protocols that are used to successfully introduce new genes. However, for some cells like stem or progenitor cells, optimization may be necessary if new genetic material is to be introduced.
The following is a protocol that has been used to introduce foreign nucleic acids into the neural progenitor cells of mice:
· Setting up a matrix in a multi-well plate - The first and most important step involves setting a matrix in a multi-well plate. This is carried out in order to determine the optimal conditions required for efficiency, gene expression as well as cell viability.
During this step, lipid to DNA ratio as well as lipid to DNA concentration is varied across the rows and columns in the plate. This technique is also useful for testing optimum transfection time, different types of lipid reagents, as well as the incubation time.
* However, in cases where this is not necessary, then the cells can first be grown in the appropriate medium for a period of between one (1) and seven (7) days.
· When ready for transfection, replace the growth medium with 0.5ml of complete growth medium (without antibiotics)
· To prepare DNA solution for each well (consisting of cells) to be transfected, dilute between 0.5 and 1.0 micrograms of the DNA with 100um reduced serum medium
· Add 2.5 microliters of PLUS reagent (used for expanding array of transfectable cells) to the DNA solution and mix gently
· Incubate the mixture for between 5 and 15 minutes at room temperature
· Following the incubation period, add between 0.1 and 2.50 microliters of lipofectamine LTX reagent (used to enhance potency balance of the cells during transfection) into the DNA solution and mix gently
· Incubate the mixture for about 30 minutes at room temperature - At the end of this step, a DNA-Lipofectamine LTX is formed
· Add about 100 microliter of the complex into each of the well and mix gently
· Incubate the cells at 37 degrees C for 18 to 24 houses in carbon dioxide
* Once the cells have been incubated for about 24 hours, they can be assessed for transgene expression. Here, researchers investigate the cells to determine whether certain characteristics are expressed.
* While transfection is mostly used to study the functions of given genes as well as products of gene expression, researchers have also been looking at the possibility of genome editing to use these cells (particularly stem and progenitor cells) for therapy/treatment purposes.
Culture
While neural stem and progenitor cells can be found in the central nervous system of adults, these cells are very few in numbers and may prove difficult to study in vivo. For this reason, culture techniques are used for the purposes of expanding their population so that they can be assessed and studied in vitro.
The following protocol is used to culture neural progenitor cells of mammals once they have been isolated:
· Once the tissue has been isolated (e.g. from the brain of a mouse) it's first put in a polystyrene dish that contains cold 1X phosphate buffer saline (PBS) using a sterile transfer pipette - Here, cutting the tissue into very small pieces is recommended.
· The tissues are then transferred into a conical tube that contains about 10mL of enzyme solution and incubated for 20 minutes at 37 degrees C. Here, the amount of buffer used in the first step should be minimized before transferring the tissue into the enzyme solution. As well, the contents of the conical tube should be mixed after every 5 minutes to enhance enzymatic digestion.
· After 20 minutes of incubation, 10lM of enzyme solution is again added to the tissue and incubated for another 20 minutes at 37 degrees C.
· Using a pipette, the enzyme solution is removed so that only the tissue is retained in the tube. 4.5mL of LI solution (light inhibitory solution) is then added, gently mixed, and discarded. This is repeated one more time and the solution again discarded.
· 6mL of HI solution (heavy inhibitory solution) is then added into the tube and the contents incubated at 37 degrees C for about 2 minutes and then discarded.
· Once the HI solution is discarded, about 5mL of NEP basal medium is added into the tube and mixed gently. Here, mixing can be achieved by lightly flicking the tube. The medium is then removed before moving to the next step.
· Once the medium (NEP basal medium) has been removed, 0.5 to 1.0ml of NEP complete medium is added and triturated about 20 times. Here, however, trituration should continue until the tissue pieces have completely dissociated.
· The cells are then incubated at 37 degrees C within about 5 percent carbon dioxide. Incubation is carried out in a humidified incubator. Neurospheres should have formed after 6 days in culture.
· Once the neurospheres form, then the medium is replaced every 3 days - Here, the neurospheres are transferred to the 15mL conical tubes and allowed to settle at the bottom of the tube at 37 degrees C. Once they have settled at the bottom of the tube, the medium has to be replaced with fresh NEP complete medium.
* Following the proliferation of neural progenitor cells during culture, the cells can be used in transfection. On the other hand, they can be placed in the appropriate culture for differentiation to occur
Markers: Neural Progenitor Cell Markers
Different types of cells express markers that allow researchers to distinguish and even classify them. Depending on the type of cells, these markers may be in the form of lipids or proteins etc.
While neural progenitors are different from neural stem cells and differentiated cells of the nervous system, they are heterogeneous which means that there are several types (subpopulations) within the nervous system.
Here, then, markers make it possible to distinguish and classify the different types of neural progenitors:
Hippocampal neural progenitor cells - Depending on the stage of development (of an individual), these cells express several markers which include:
- Glial fibrillary acid protein
- Sex determining region Y-bx 2 (SOX2)
- Brain lipid-binding protein
- Nestin
Proliferative neural progenitors - Capable of self-renewal through symmetric division.
Some of the markers associated with these cells include:
- SOX2
- Paired box protein Pax-6
Intermediate progenitors and early immature neurons - These cells can be found in the cerebral cortex where they undergo development.
Some of the markers associated with these cells include:
- Doublecortin (DCX encoded by the DCX gene)
- Polysialylated-neural cell adhesion molecule (PSA-NCAM)
Functions
As mentioned, there are several types of neural progenitor cells with the ability to proliferate and differentiate to give rise to several types of cells in the central nervous system. Because of their variety, these cells can be unipotent - only capable of differentiating to give rise to one type of cell in the nervous system; bipotent - can differentiate to give rise to two types of cells in the nervous system; or multipotent - capable of giving rise to several types of cells in the nervous system.
While there are many studies looking on how to manipulate and enhance the functions of neural progenitors, they are primarily involved in neurogenesis where they give rise to different types of cells (neurons and glial cells) in the central nervous system.
Their ability to differentiate and produce different types of cells in the nervous system has been shown to decline with age.
See Also: Hematopoietic Progenitor Cells, Endothelial Progenitor Cells
Return to Progenitor Cells main page
Return to Stem Cells main page
Return to understanding Cell Division
Return to Nerve Cells main page
Return from Neural Progenitor Cells to MicroscopeMaster.com
References
Arnold R Kriegstein, Stephen Noctor, and Veronica Martinez Cerdeno. (2006). Patterns of neural stem and progenitor Cell division may underlie evolutionary cortical expansion.
Atsunori Shitamukai and Fumio Matsuzaki. (2012). Control of asymmetric cell division of mammalian neural progenitors.
Catarina CF Homem, Marko Repic, and Juergen A Knoblich. (2015). Proliferation control in neural stem and progenitor cells.
Minde I. Willardsen Brian A. Link. (2011). Cell biological regulation of division fate in vertebrate neuroepithelial cells.
Tara Walker, Jeffrey Huang, and Kaylene Young. (2016). Neural Stem and Progenitor Cells in Nervous System Function and Therapy.
Links
https://www.axolbio.com/page/transfection-of-neural-progenitor-cells
Find out how to advertise on MicroscopeMaster!