Natural Killer Cells
Definition, Action, Cancer and Normal Range
Definition
Discovered in 1975 by Rolf Keissling, natural killer cells (NK cells) are lymphocytes that primarily target virus-infected cells (and other pathogen-infected cells) as well as cancerous cells.
Being members of the innate immune system, they are part of the early line of defense and thus respond within a few hours of cell invasion.
As compared to the other lymphocytes (B cells and T cells), natural killer cells are generally larger in size and make up between 5 and 15 percent of the total lymphocytes in circulation.
While the majority of these cells can be found in such regions as the spleen, bone marrow, lymph nodes, and peripheral blood, they can also be found in various primary and secondary compartments.
In addition to releasing contents of cytotoxic granules that destroy the target cells (they are cytotoxic cells), natural killer cells also function by releasing several cytokines that regulate the functions of other immune cells.
Some of the signaling molecules secreted by natural killer cells include:
- TNF-α (Tumor necrosis factor-α)
- IFN-γ - (Interferon-γ)
- Chemokines (1-5 and CXCL8)
- GM-CSF - (granulocyte macrophage colony-stimulating factor)
Action of Natural Killer Cells (NK Cells)
Like the other lymphocytes, natural killer cells originate from the hematopoietic progenitor cells (HPCs) through the lymphoid lineage. As mentioned, natural killer cells make up part of the innate. In this case, then, there is no specificity as is the case with B and T cells that specifically respond to given antigens.
Despite the fact that natural killer cells originate from a common progenitor as B and T cells, they are different in that they are not part of the adaptive immune system. Here, the biggest difference between the natural killer cells and the other two (B and T cells) is the fact that natural killer cells do not have antigen receptors.
* Unlike T cells, which have CD8, a co-receptor for the T-cell receptor, the majority of natural killer cells express CD56 which allows these cells to be divided into two based on the type of CD56 they possess.
While natural killer cells belong to the innate immune system and are therefore not as specific as the other lymphocytes, it's worth noting that they have several receptors through which their actions are regulated.
When the activating receptor receives kill signals, the cell is activated to act in response to cell invasion by a foreign microorganism - so that the target cells can be destroyed.
In general, receptors of natural killer cells are divided into two main groups that include:
· Activating receptors - Characterized by ITAM (immunoreceptor tyrosine-based activation motif)
· Inhibitory receptors - Characterized by ITIM (immunoreceptor tyrosine-based inhibition motif)
The following are modes of action of natural killer cells based on the type of signal they receive:
In addition to the peripheral blood, where they make up between 5 and 15 percent of the total lymphocytes, natural killer cells can also be found in the uterus, bone marrow, the liver, spleen, and lungs among other lymphoid tissues during their development. As such, they are well-positioned to respond to infected cells as well as cancer cells.
Generally, the cytotoxicity action of these cells is divided into three main phases that include:
Target cell identification - As already mentioned, natural killer cells are not as specific as some of the other lymphocytes. Therefore, given that they attack various cells indiscriminately, there has to be a regulatory mechanism in place to ensure that they do not interact and destroy healthy, normal cells of the body.
Based on many molecular studies, natural killer cells were shown to have many surface receptors that receive signals and influence the actions of these cells. Activation receptors (expressed by the NK cells) are capable of binding to a range of proteins located on the surface of healthy cells and can activate natural killer cells to release chemicals that would destroy the cell.
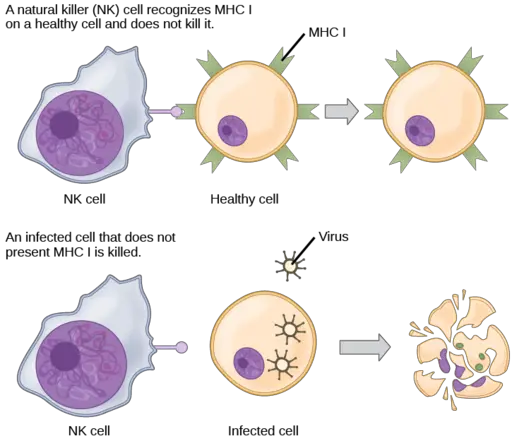
In addition to activation receptors, however, natural killer cells also have inhibitory receptors on their surface. When these receptors identify MHC Class I (major histocompatibility complex class I), then the cell is inhibited. Upon binding to the MHC Class I molecules, the cell receives negative signals that turn off the lytic capacity of natural killer cells.
* In mice, Ly49s, which is a type II glycoprotein, have been shown to identify MHC Class I molecules which in turn prevents the NK cells from destroying healthy cells. In human beings, receptors that identify and interact with the MHC molecules mostly belong to the Killer immunoglobulin-like receptor family (KIR)
As compared to the normal, healthy cells, infected cells (by viruses or bacteria) tend to lose their ability to express MHC class I molecules on their surface. As well, bacteria, among other pathogens that invade cells, do not express MHC Class I molecules.
In a case where a cell is infected by virions (which replicate their DNA), normal cellular activities are affected and such cells have also been shown to be unable to express MHC class I. When the natural killer cells identify various surface molecules on the surface of these cells but fail to identify/detect the major histocompatibility complex class I, then the cell becomes a target cell and has to be destroyed.
Here, the activation receptors of the natural killer cells are activated but the inhibitory receptors of these cells are not activated to inhibit the lytic capacity of the killer cells. Therefore, activation outweighs inhibition causing the cell to become a target.
* In a case where the infected continue expressing MHC class I molecules, this capacity is significantly reduced which means that inhibition signals that natural killer cells will receive are far less compared to the activation signals.
* Natural killer cell receptors recognize the ligands located on the surface of cells that have been infected by pathogens, those that are stressed or cells that have been transformed.
A good example of these receptors is the Fcy RIII receptor (which is an Fc receptor) that recognizes the Fc part of antibodies associated with some unhealthy cells.
Target cell contact and the formation of an immunological synapse - The next and last phase of natural killer cell action involves the destruction of the target cell. Following target cell recognition, the next phase of NK action involves the formation of a cell-cell junction with the target cell.
The formation of this junction between the target cell and a natural killer cell is particularly important given that it ensures that only the target cell is destroyed and not the other healthy, neighboring cells.
For natural killer cells to adhere to the target cells, studies have shown that signaling by β2-integrin LFA-1 (lymphocyte function-associated antigen-1) is necessary. Here, the integrin (LFA-1) first identifies the ICAM-1 (Intercellular Adhesion Molecule 1) located on the target cells. It then binds to the ICAM-1 resulting in the production of signaling molecules that activate the formation of the synapse.
During synapse formation, the LFA-1, along with other adhesion molecules have been shown to settle at the periphery of the synapse. Here, they not only promote adhesion but also activate signals involved in the accumulation of F-actin.
Through the polymerization of the actin as well as its accumulation at the synapse, it also contributes to the formation of the activation synapses in addition to promoting the cytotoxicity of natural killer cells.
Learn more about cell adhesion here.
* In general, formation of the cell-cell junction is said to start with engagement of various transmembrane receptors with the ligands located on the cell surface. As a result, an intercellular junction starts forming and this is known as the immunological synapse. This synapse is particularly important as it's the bridge through which signals pass and influence the consequent outcomes.
Natural cell-induced target cell death - The last phase of natural killer cells results in the destruction of the target cell (infected, modified, or stressed cell) and is also known as degranulation. In some books, the degranulation process starts with the formation of the immunological synapse.
Following the docking process, perforin from NK Cells granules is released on the target cell. The cytotoxic protein (perforin) then undergoes polymerization and forms pores on the membrane of the target cell. This allows granzymes (serine proteases also found in the granules) to gain entry into the cell and activate caspase molecules involved in apoptosis of the cell.
Cancer: Response of Natural Killer Cells to Cancer Cells
Like virally infected cells, studies have shown some cancer cells to either lack or downregulate MHC class I molecules. In other cases, these cells have also been shown to upregulate such ligands as MHC class I-related chain A and B ligands. As such, they are unable to provide sufficient inhibitory signals that would otherwise prevent them from being destroyed.
As mentioned, healthy cells express various signaling molecules on their surface as well as MHC class I molecules. Here, the MHC class I molecules play an important role in preventing the destruction of the cell by natural killer cells.
Given that cancer cells are unable to express enough MCH class I molecules, they cannot send enough inhibitory signals that would turn off the lytic capacity of natural killer cells. The increased expression of activation ligands (e.g. MHC class I-related chain A and B) activates natural killer cells to target and destroy these cells.
* In a tumor microenvironment, some studies have shown cancer cells to be capable of evading natural killer cell responses through increased expression of MHC class I molecules while also decreasing the expression of NKG2D ligands.
Apart from the ability of some cancer cells to evade natural killer cells, some cancer patients have been found to have functionally impaired natural killer cells. For this reason, they are unable to effectively target and destroy cancer cells.
In the medical field, this has led to the development of various compounds aimed at enhancing the activities of these cells. However, as is the case with virally infected cells, NK cells can also destroy cancer cells through cytotoxic activities.
Here, the cells first identify abnormal cells through the ligands expressed on their surface before forming a synapse. This allows the cell to release granular contents that stimulate apoptosis. These contents create holes on the cell membrane of cancer cells which causes them to swell and burst.
Normal Range
As mentioned, natural killer cells make up between 5 and 15 percent of the total lymphocytes in circulation. The normal range of natural killer cells varies between the different stages of life (a few days after birth to over 65 years old).
For babies, from a few days old to about 23 months old, the normal range of these cells varies from 55 to 4000 cells per microliter of blood. For instance, in a newborn (between 1 and 6 days), the number of NK cells has been shown to vary between 500 and 3,100 cells per microliter of blood.
For a baby between 15 and 23 months, this number changes from 55 to 4,000 cells per microliter of blood.
For a teenager, between the ages of 10 and 16 years, the normal range has also been shown to vary from 92 and 1,200 cells per microliter of blood. However, this changes to between 78 and 470 cells per microliter of blood for individuals between the ages of 16 and 64.
Immune System: Role of Natural Killer Cells in Adaptive Immune Response
As cells of the innate immune system, natural killer cells are some of the first cells to respond to infected cells. However, they have also been shown to play an important in regulating the adaptive immune system by regulating the manner how some of these cells respond.
The following are some of the relationships between natural killer cells and other cells of the immune system:
Dendritic Cells and Natural Killer Cells
Like B cells, dendritic cells (DC) are antigen-presenting cells. As such, they play an important role in processing and presenting antigen material to the T cells thus activating their functions.
Early on during development, dendritic cells and natural killer cells have been shown to influence each other’s immune actions. Whereas dendritic cells are involved in the activation of natural killer cells, the natural killer cells can either promote the maturation of dendritic cells or kill immature forms.
Through their interaction with natural killer cells, dendritic cells have been shown to influence the release of cytokines by the natural killer cells which promotes their proliferation and cytolytic activity.
Plasmacytoid dendritic cells, for example, release type 1 IFN (interferon) which promotes the cytotoxic activities of natural killer cells. Dendritic cells also produce IL-15 (interleukin 15), a cytokine that promotes the development of natural killer cells.
Natural killer cells influence the activities of dendritic cells through IFN-γ and tumor necrosis factor (TNF). Through the release of high amounts of both TNF and IFN-γ, studies have shown natural killer cells to promote the maturation of dendritic cells.
Here, TNF contribute to the expression of co-stimulatory molecules on dendritic cells while the combination of TNF and IFN-γ activate their capacity to produce IL-2 (interleukin 2).
Macrophages and Natural Killer Cells
Macrophages are cells of the immune system that engulf and destroy damaged or dead cells. As is the case with dendritic cells, studies have shown macrophages and natural killer cells to influence each other.
With the help of surface receptors, macrophages are able to identify, bind, and engulf invading pathogens. As a result, macrophages are stimulated to release cytokines (e.g. IL-12) that in turn promote the entry of natural killer cells into tissue.
The natural killer cells are involved in the destruction of infected cells as well as cells that send stress signals. Natural killer cells release type II interferon, a primary cytokine that plays a role in the action of macrophages. Therefore, the two types of immune cells act by creating a positive feedback that enhances their respective actions in tissue which prevents infections from spreading.
T Cells and Natural Killer Cells
While both the natural killer cells and T cells have a common origin, natural killer cells belong to the innate immune system while T cells are cells of the adaptive immune system. However, natural killer cells are involved in the activation of T cells through the production of cytokines.
Cytokine production by natural killer cells has been shown to contribute to T cell priming (first contact between the precursor of antigen-specific T-helper cell and an antigen) as well as the resulting differentiation.
The expression of OX40 ligand among other co-stimulatory molecules by natural killer cells promotes the proliferation of T cells. In the natural killer cells-macrophage relationship, type II interferon produced by natural killer cells activates development of macrophages which in turn results in the production of cytokines that activate T cells.
Consequently, activation of T cells activates the adaptive immune response while also downregulating activities of natural killer cells.
See also: Immunology as a field of study
Return from Natural Killer Cells to MicroscopeMaster home
References
Alex M. Abel. (2018). Natural Killer Cells: Development, Maturation, and Clinical Utilization.
Guido Ferlazzo and imageBarbara Morandi. (2014). Cross-talks between natural killer cells and distinct subsets of dendritic cells.
Iona S. Schuster et al. (2016). “Natural Regulators”: NK Cells as Modulators of T Cell Immunity.
Norberto Walter Zwirner and Andrea Ziblat. (2017). Regulation of NK Cell Activation and Effector Functions by the IL-12 Family of Cytokines: The Case of IL-27.
Sourav Paul and Girdhari Lal. (2017). The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy.
Links
Find out how to advertise on MicroscopeMaster!