Mycoplasmas
Examples, Characteristics, Infection and Treatment
Definition: What are Mycoplasmas?
Mycoplasmas are small spherical/pear-shaped bacteria that can exist as saprophytes or parasites.
Apart from being some of the smallest bacteria on earth, Mycoplasma species also lack a cell wall around the cell membrane which sets them apart from other bacteria (most of which have a cell wall).
Given that they exist as free-living organisms or parasites of animals and plants, they are widespread in nature and can be found in aquatic and terrestrial environments.
Currently, over 120 species of the genus Mycoplasma have been identified and described with Mycoplasma pneumoniae, responsible for upper and lower respiratory infections, being the most common species in the group.
* Mycoplasmas were previously referred to as pleuropneumonia-like organisms (PPLO).
Examples of other Mycoplasmas include:
- Mycoplasma hominis
- Mycoplasma genitalium
- Mycoplasma mycoides
- Mycoplasma salivarium
- Mycoplasma capricolum
- Mycoplasma arginini
- Mycoplasma canis
Classification of Mycoplasmas
Kingdom: Bacteria - As members of the kingdom Bacteria, Mycoplasmas are prokaryotic single-celled organisms. However, they are different from other bacteria in that they lack a cell wall.
Phylum: Firmicutes - Mycoplasma are classified under the Phylum Firmicutes which consists of Gram-positive bacteria. However, some members of this division appear as Gram-negative bacteria after staining because of the characteristics of their outer membrane.
See page on Gram positive and Gram negative bacteria
Class: Mollicutes - The name Mollicutes is derived from the Latin word "Mollis" which means soft. Members of this group, such as Mycoplasmas lack a cell wall and tend to be very small in size.
Order: Mycoplasmatales - The order Mycoplasmatales consists of Mycoplasma and Ureaplasma species that contain a small genome.
Family: Mycoplasmataceae - The family Mycoplasmataceae is made up of the genera Mycoplasma and Ureaplasma. In this family, the majority of species are sexually transmitted. Their shape varies from filamentous to spherical depending on the species - However, some of the species have been shown to change shape under certain conditions.
Genus: Mycoplasma - Characteristics of the genus Mycoplasma are discussed below.
Ecology and Distribution
Mycoplasma infections have been reported in different regions across the world which is evidence that these bacteria are widely distributed across the globe.
As parasites, they infect a variety of hosts including reptiles, mammals, fish, and arthropods. As such, they can be found in both terrestrial and aquatic environments in which they infect these hosts.
Apart from animals, some species are parasites of different plant species. The list of hosts that harbor these organisms, however, is reported to be increasing as the number of identified species rises.
Saprophytic species and strains, on the other hand, have been isolated from a number of habitats including sewage, manure, soil, and humus among others. In order to continue replicating, however, these species live in intracellular and extracellular environments where they depend on the fragments of dead or living cells.
* Among infected human beings, the bacteria may reside in the mucosa of the upper respiratory tract, the oral cavity, or urogenital tract.
Characteristics
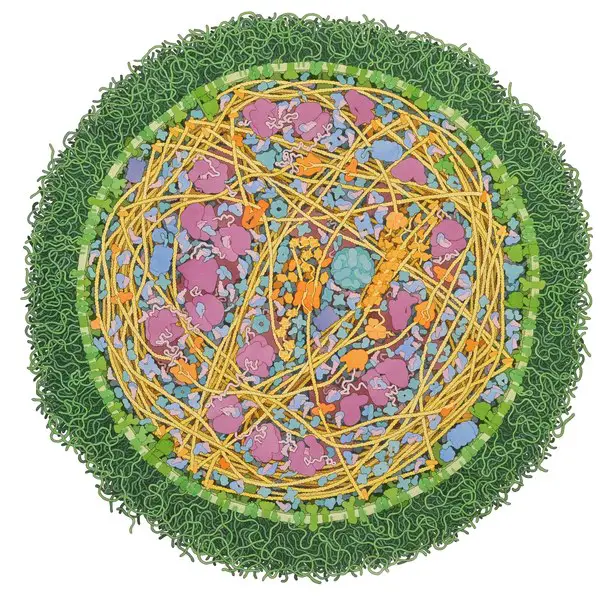
Morphology and Cell Structure of Mycoplasmas
For the most part, Mycoplasmas are spherical in shape with their size varying between 0.3 and 0.8um in diameter. This not only makes them the smallest bacteria but the smallest cells in general. While the majority of species exhibit a spherical shape, some may appear pear-shaped or flask-shaped with branching filaments of varying lengths.
Unlike many of the other bacteria that have a cell wall, Mycoplasmas do not. For this reason, the general shape of the organism is maintained by the cytoskeleton in its structure.
Using detergent treatment, researchers have been able to visualize the network of filament threads and rods that make up this cytoskeleton. Based on microscopic studies, Mycoplasma cells have been shown to consist of three main organelles.
These include:
* The genome of Mycoplasmas is about 800kb (consisting of about 816,394 base pairs) in size with a G+C content that averages 40.0mol percent.
Cell Membrane
Through early electron microscopic studies, Mycoplasmas were found to lack both the cell wall and intracytoplasmic membranes. However, these studies also showed that the cell is surrounded by the plasma membrane. This membrane has been isolated using osmotic lysis which has allowed researchers to study associated characteristics (chemical, antigenic, and enzymatic properties).
For the majority of species, the cell membrane is made up of between 60 and 70 percent proteins and 20 to 30 percent lipids. In infected hosts, Mycoplasmas have also been shown to acquire large amounts of sterols from the host and incorporate it into their plasma membrane. The sterols are then used for a number of functions including regulating membrane fluidity with changes in temperature etc.
Cytoskeleton
With the absence of the cell wall in Mycoplasmas, the cytoskeleton/cytoskeleton-like structures have been suggested to modulate the shape of the cell.
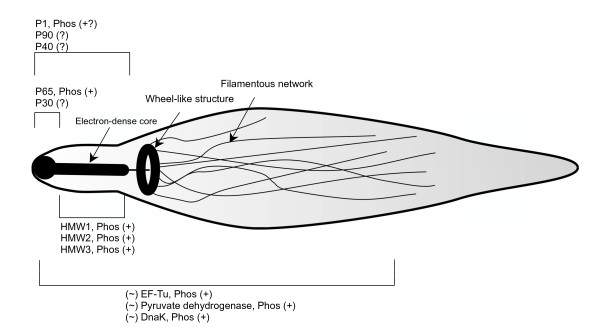
In Mycoplasma pneumoniae, the cytoskeleton, also referred to as the Triton shell, consists of a thick rod as well as a network of filaments which produces a basket-like structure. Here, the relatively thick rod, which is made up of striated bundles of filaments, provides support to the attachment organelle with the basket-like structure providing structural support for the cell as a whole.
The cytoskeleton also consists of a number of proteins which include:
· P1 adhesin - Allows the organism to bind to the cell of the host and other surfaces
· Proteins that provide support for P1 adhesin
· HMW1 and HMW2 - Involved in the formation of attachment organelles
· Proteins that localize at the proximal end of the EDC
· HMW3, P65, and P30 - Also localized at the attachment organelles
Motility
Motility in Mycoplasmas is made possible by tiny (less than 50nm in length) leg-like proteins located on the cell membrane. In particular, these proteins originate from a nose-like anterior projection. Using these proteins, suspected to be protein Gli349, the organism is also able to adhere, detach and reattach the organism on to various surfaces where they have been shown to move at speeds of between 2 and 4.5 micrometers a second.
The energy required for movement is obtained from ATP hydrolysis. Here, however, it's worth noting that by gliding, Mycoplasmas are only capable of moving forward and never in reverse.
Apart from the leg-like proteins, the nose-like protrusion also consists of various cytoskeletal structures. These structures make up the hexagonal-lattice which is located at the tip of the protrusion where it forms a hemispherical cap that measures about 235nm in width and 155nm in length.
This hemispherical cap is in turn attached to a number of flexible proteins that are located in the cytoplasm. These proteins, which are flexible with a tentacle-like appearance, are attached to particles (20nm in size) which have been suggested to attach the leg-like proteins to the tentacles.
Nutrition
The majority of Mycoplasmas exist as parasitic or as commensals. As such, they require a host for survival. Unlike a number of other bacteria, however, Mycoplasmas are capable of fermenting available materials in order to produce ATP. This, then, makes them independent given that they are capable of generating their own source of energy.
While they are dependent on their hosts for various metabolic functions (because they have lost their use of the electron transport chain), respiration is achieved through fermentation (anaerobically) without the use of the ETC.
Currently, seven species of Mycoplasma are known to be human pathogens.
These include:
- M. penetrans
- M. pneumoniae
- M. urealytium
- M. hominis
- M. genitalium
- M. pirum
- M. fermentation
As parasites, species like M. pneumoniae have to attach to the cell of the host first. This process involves the use of adhesion auxiliary proteins as well as a network of adhesion systems. Following contact with the target cell (this includes a variety of cells such as red blood cells, HeLa cells, Fibroblasts, and even macrophages) the precursor or P1 proteins have been shown to rapidly shift to the apical region where they are involved in the production of P1 proteins involved in the attachment.
Apart from these proteins, Mycoplasmas also use a number of other proteins including P30 adhesion factor-related protein and HMW 1–5 polypeptides among others.
Following adherence, the microtubules of the parasite extend and penetrate the cell of the host. This allows the organism to obtain various materials including cholesterol, glucose, and amino acids among others. In the process, this causes damage to the cells.
In some cases, the Mycoplasma invades the cell where it may reside in the cytoplasm or the nucleus which also results in cell damage. As intracellular or extracellular parasites, M. pneumoniae has also been shown to cause damage by releasing such toxins as exotoxins and other exotoxin-like substances.
Adaptations
For Mycoplasma, lacking a cell wall has a number of benefits that contribute to its survival. For instance, because they only have a plasma membrane, this allows such parasitic species as M. bovis to alter their shape and thus optimize efficiency within the host.
In vivo, they can change shape from spherical to a filamentous and fried-egg appearance. This also allows them to adapt to different environments. While Mycoplasmas are generally extracellular organisms, they can invade the cell and reside in the cytoplasm or nucleus.
As well, the lack of cells allows Mycoplasmas to evade the actions of many antibiotics. For the most part, many of the antibiotics used against bacterial cells destroy them by targeting the cell wall. Given that Mycoplasma do not have a cell wall, these antibiotics are ineffective against them.
For this reason, such Mycoplasma parasites as M. genitalium have exhibited antibiotic resistance to such antibiotics as macrolides. With other species of Mycoplasma being discovered, it has become important to work towards antibiotics that specifically target these parasites. See also: How do antibiotics kill bacteria?
Apart from the ability of these organisms to change their shape, pathogens like M. bovis are also able to change the proteins located on their surface. This makes it challenging for the immune system of the host to effectively mount an attack against these parasites and destroy them.
Reproduction
Reproduction in Mycoplasmas occurs through binary fission and budding. Binary fission starts with DNA replication which begins at the site near the dnaA gene. Following replication, the chromosomes migrate to opposite poles of the cell before the cell divides thus ensuring that each of the daughter cells contains the DNA material.
Following cell division, each of the daughter cells contains genetic material of the parent as well as cytoplasm and ribosome. In cases where replication is inhibited, the cells have been shown to start branching.
In some cases, the bacteria produce elementary bodies that form as buds on the surface of the parent cells. These elementary bodies, which are less than 180nm (some can be as large as 400nm in diameter) in diameter resemble virus particles that are infectious and allow the life cycle of the pathogen to continue.
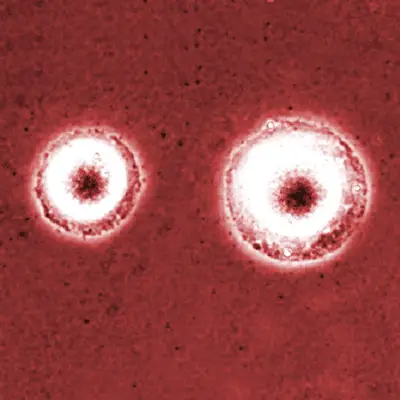
Microscopy
Ranging from 0.3 to 0.8um in diameter, Mycoplasmas are too small to be detected by a light microscope. For this reason, Mycoplasma culture techniques are often used to grow colonies that can then be observed using an inverted microscope.
Culture Technique
To culture Mycoplasma, 0.1 to 0.2 ml of the cell suspension (antibiotic-free cell suspension) is inoculated onto the surface of a Mycoplasma agar plate. The plate is then incubated for 28 days at 37 degrees C in an environment that is enriched with 5 percent carbon dioxide.
Observation
When the plate is viewed under an inverted microscope at low magnification (x4 and x10), Mycoplasma colonies can be seen presenting a fried-egg morphology - They look like a fried egg with a darker spot at the center.
Infections and complication associated with the pathogenicity of Mycoplasma species include:
Sexually transmitted infections - Mycoplasma genitalium is not part of the normal virginal flora. In the event of an infection, it causes urinary and genital tract infections and can be sexually transmitted. As a result, it affects both men and women.
Infertility - In men, pathogenicity of Mycoplasma hominis has been associated with genital inflammation and male sterility.
Infant mortality - Given that Mycoplasmas can infect the reproductive system (as perinatal pathogens), the infection can be transmitted to the baby which can affect their health.
Some of the other complications include:
- Encephalitis
- Optic neuritis
- Cranial nerve palsies
- Aseptic meningitis
Return from Mycoplasmas to MicroscopeMaster home
References
Jun He et al. (2016). Insights into the pathogenesis of Mycoplasma pneumoniae.
Lesley Young, Julia Sung, Glyn Stacey & John R Masters. (2010). Detection of Mycoplasma in cell cultures.
Shmuel Razin. (1996). Mycoplasmas. Medical Microbiology. 4th edition.
Shmuel Razin and Leonard Hayflick. (2010). Highlights of mycoplasma research-An historical perspective.
Shmuel Razin, David Yogev, and Yehudith Naot. (1998). Molecular Biology and Pathogenicity of Mycoplasmas.
Links
https://cronodon.com/BioTech/bacteria_mycoplasmas.html
Find out how to advertise on MicroscopeMaster!