Leydig Cells
Location, Function, Secretion and Vs Sertoli Cells
Overview
Leydig cells are testicular cells involved in the production of androgens as well as the development of secondary sex characteristics.
Location
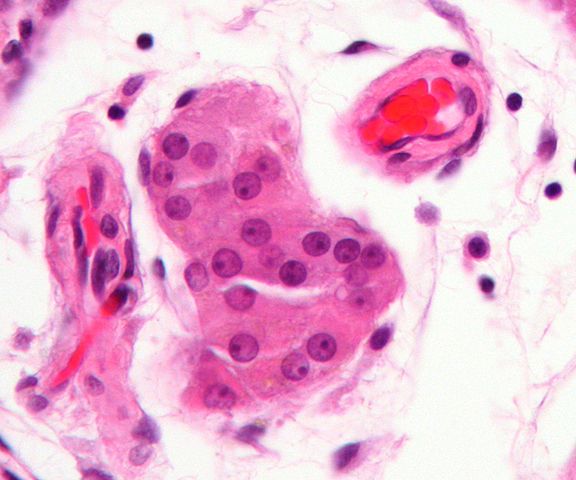
In animals, Leydig cells are normally located within the connective tissue that surrounds the seminiferous tubules (where Sertoli cells reside). As such, they are also commonly referred to as interstitial cells. Here, however, they have been shown to have two main phases of growth with each phase producing a different population of Leydig cells.
The first population of Leydig cells is known as fetal Leydig cells (FLC). This phase is divided into three main stages that include the differentiation stage, maturation, and involution stage. Early on in the fetal stage of development, the activation of Sry-Sox9 genetic cascade causes Sertoli cells to start differentiating.
Consequently, Sertoli cells influence the male gonads to develop into testis. In the seminiferous tubules, these Sertoli cells also secrete desert hedgehog (Dhh - A protein that is encoded by DHH gene) and platelet-derived growth factors that influence the differentiation of fetal Leydig cells.
In mice, these cells have been shown to reside in the interstitial region in the testis. Given that they are incapable of mitosis, this increase is suspected to result from the progenitor cells.
* During the fetal phase, differentiation of Leydig cells takes place during the gestation period (7 to 14 weeks). This is followed by maturation which occurs during the gestation period (14 to 18 weeks). Following maturation, involution continues until the child is born.
* The fetal Leydig cells are characterized by numerous droplets of lipids.
Fetal Leydig cells have several functions depending on the organism. For instance, while they produce testosterone in rats between the 15th day of gestation and before birth, they have been shown to produce androstenedione in mice.
In some organisms, some of these cells may persist in adulthood (during the adult life of the organism). However, in such cases, they only produce a very little amount of testosterone. In most animals, however, this population of Leydig cells disappears once the individual is born.
The differentiation and development of adult Leydig cells start after the baby is born. In mice, for instance, postnatal differentiation and development of Leydig cells have been shown to start 2 to 3 weeks after birth.
While some researchers initially believed that these cells were dependent on the fetal Leydig cells, they have been suggested to originate from stem cells. However, early on during the differentiation of adult Leydig cells, fetal Leydig cells the fetal Leydig cells appear as smaller cells that are still undergoing regression.
* The adult Leydig cells persist over the adult life of an individual.
* As compared to the fetal Leydig cells, adult Leydig cells are characterized by a smaller number of lipid droplets.
Interstitial compartment - As mentioned, Leydig cells are located in the interstitial compartment. It makes up the outside part of the testis cords and consists of a number of other cells.
For instance, in both human beings and rodents, the interstitial compartment has been shown to contain steroidal Leydig cells, vascular smooth muscle cells, vascular endothelial cells, and immune cells among others.
Functions
Functions of Fetal Leydig cells
Although fetal Leydig cells are lost before adult Leydig cells start developing, they are involved in the development of the male reproductive tract (male sexual characteristics) in the embryo. Along with fetal Sertoli cells, fetal Leydig cells have been shown to produce androgens. A process called steroidogenesis.
This has been associated with a number of outcomes including sexual dimorphism of the brain, development of the Wolffian duct (to form the epididymis, the vas deferens, and seminal vesicles), as well as the development of the male genitalia among others.
As such, fetal Leydig cells are essential in the development of male characteristics in the embryo. In the brain, androgens produced by both Sertoli cells and fetal Leydig cells are suspected to influence subtle patterning and given sexual behaviors that will become evident in the adult.
In addition to androgens, fetal Leydig cells also produce insulin-like factor 3 that regulates the descent of the transabdominal and inguinoscrotal testis. Deficiency of these secretions in the embryo has been shown to result in cryptorchidism (where testes are retained in the body cavity) and infertility in adults.
Here, the production of insulin-like factor 3 (INSL3) by fetal Leydig cells influences the differentiation of the gubernaculum ligament which is then responsible for regulating the transabdominal phase of testicular descent.
This is particularly important in that the process allows the testicles to descend towards the scrotum where the temperature range (with an optimum temperature of 35 degrees C) is ideal for spermatogenesis. In the body cavity, in the case of cryptorchidism, higher temperature (37 degrees C) is detrimental to sperm production thus resulting in infertility.
* Cryptorchidism has been associated with germ cell tumors as well as Leydig cell tumors.
* Based on recent studies, fetal Leydig cells have been shown to be involved in the proliferation of Sertoli cells
Functions of Adult Leydig Cells
Like fetal Leydig cells, adult Leydig cells are also involved in the production of insulin-like factor 3. Along with testosterone, this peptide has been shown to play an important role in maintaining fertility in adult males.
Based on a number of studies, a lowered concentration of Insulin-like factor 3 has not only been linked to Leydig cell dysfunction in adults but also infertility. For young boys about to hit puberty, studies have shown there to be a significant rise in Insulin-like factor 3 levels proving that the Leydig cells play an important role in fertility.
* The concentration of Insulin-like factor 3 continues to decline between the ages of 35 and 80.
Adult Leydig cells are primarily involved in the production of testosterone under the influence of Luteinizing hormone (LH). Based on research studies conducted in the 1960s, the stimulation of rabbit testis with Luteinizing hormone resulted in the production of testosterone.
Follow-up studies revealed that interstitial cells metabolized cholesterol in order to produce pregnenolone with progesterone ultimately being transformed into testosterone.
Here, experiments involving seminiferous tubules did not yield similar results which led to the conclusion that it's the cells located in the interstitial tissue that are responsible for testosterone production - These cells are today known as Leydig cells.
Testosterone - A sex hormone that contributes to the development of reproductive functions in men (as well as most male animals). Although studies have shown women to also produce this hormone, it's produced at very low levels compared to men.
In men, the hormone is involved in the following functions (related to sexual characteristics in male animals):
- Increased size of the penis and testes
- Growth of chest, armpit, and pubic hair
- Maintaining sex drive
- Sperm production and muscle strength
- Deepening of the voice during puberty
* After 30 to 35 years of age, the level of testosterone has been suggested to decline by about 1 percent each year.
* Abnormally low testosterone levels have been associated with increased breast size, hair loss, hot flashes, reduced libido, and reduced muscle mass, etc.
Secretion
As mentioned, Leydig cells are involved in the production of androgens involved in the development of male sexual characteristics. Given that the section above has already covered testosterone, this section will focus on two other androgens.
These include:
Androstenedione - Produced by fetal Leydig cells before it's converted to testosterone by HSD17B3, a gene found in Sertoli cells as well as fetal Leydig cells themselves.
Although androstenedione is initially produced in the fetal stage, with secretion peaking around the 16th week, studies have shown increased production of Luteinizing hormone (LH) at the 18th to 20th week resulting in the production of testosterone rather than androstenedione.
Dehydroepiandrosterone - Like androstenedione, dehydroepiandrosterone (DHEA) is also produced by Leydig cells through a process known as steroidogenesis. As an intermediate that is produced during the production of steroid hormones, dehydroepiandrosterone is ultimately converted into testosterone as well as estradiol in the target tissues.
In a study to determine the role of dehydroepiandrosterone in rats, the findings showed that in the sample treated with dehydroepiandrosterone, there was a marked increase in serum testosterone and estradiol.
Although Leydig cells are involved in the production of dehydroepiandrosterone, it's worth noting that this largely involves biotransformation to produce the molecule.
In human beings, dehydroepiandrosterone has been shown to have a number of positive effects that include:
- Preventing obesity
- Limiting the development of cancerous cells
- Preventing atherosclerosis
* Dehydroepiandrosterone is also produced in the adrenal gland, ovaries as well as the brain in small quantities. In women, it plays an important role in producing oestrogens (about 75 percent of the total oestrogens before menopause and 100 percent of oestrogens after menopause).
* As mentioned, conversion of dehydroepiandrosterone also results in the production of oestradiol. It's a steroid hormone and the main oestrogen in women. Among other functions, oestradiol plays an important role in the maturation and maintenance of the reproductive system in women.
Leydig Cells vs. Sertoli Cells
Like Leydig cells, Sertoli cells are also found in the testes where they are involved in the sexual reproductive system. Although the two types of cells are involved in the reproductive system, they have a number of differences.
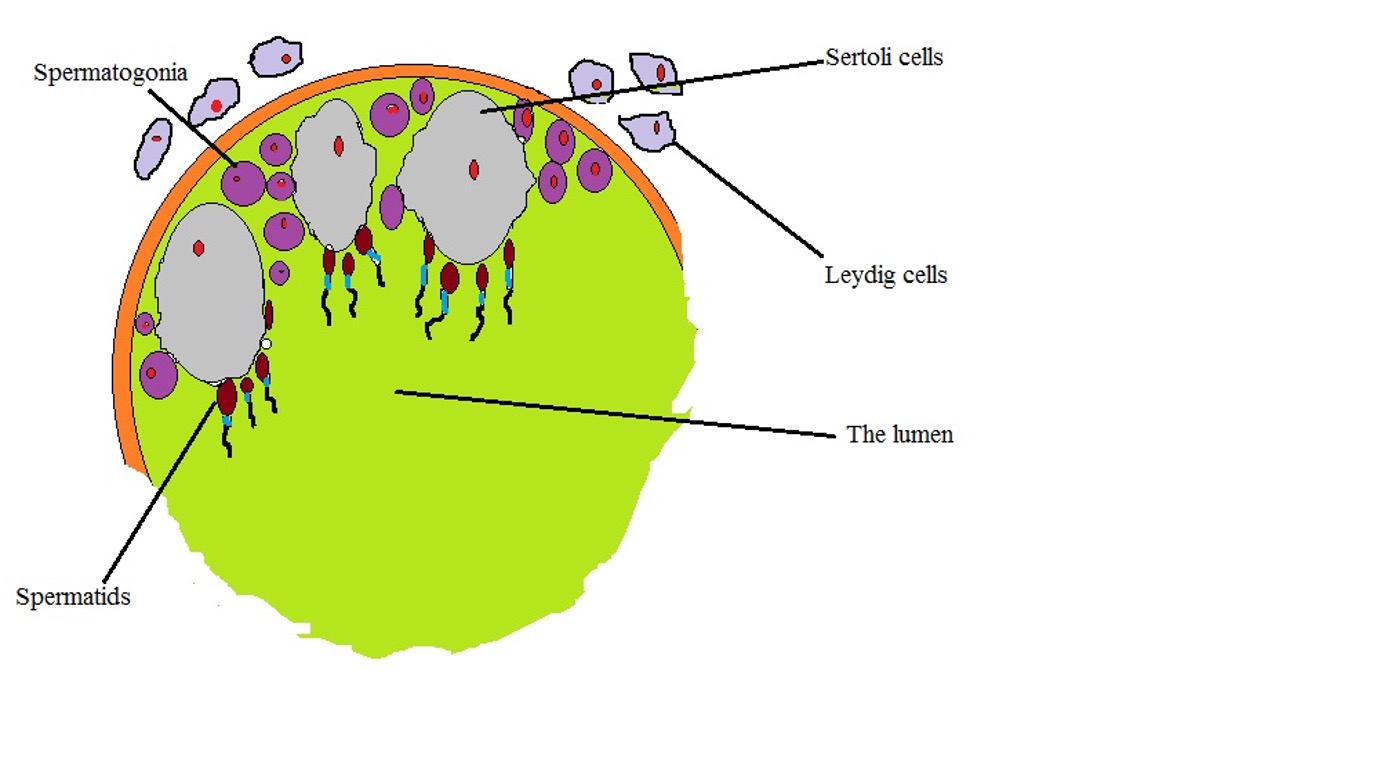
One of the biggest differences between the Leydig cells and Sertoli cells is with regard to their specific location in the testis. Here, Sertoli cells are located in the seminiferous tubules which are also the site of germination for germ cells.
Here, Sertoli cells are arranged in a manner that forms the blood-testis barrier thus preventing the movement of given substances across the epithelium formed by Sertoli cells. This is particularly important in that it provides protection for germ cells from various agents in circulation while also preventing the movement of various antigenic material (produced as germ cells mature) from entering the bloodstream (otherwise, this would create an autoimmune response against germ cells).
Leydig cells, on the other hand, are located in the interstitial tissue; adjacent to the seminiferous tubules. As such, they are also referred to as interstitial cells.
Morphologically, several differences can also be identified between the two types of cells. For instance, in the seminiferous tubules, Sertoli cells have been shown to be elongated with long cytoplasmic arms that wrap around the germ cells. This allows Sertoli cells to interact and nurture germ cells as they differentiate.
In addition, they have a large nucleus that has been shown to change in shape under certain circumstances. Leydig cells exhibit a polyhedral shape. In culture, however, they may display different shapes ranging from spindle and triangular shape to polygonal and other irregular shapes. However, like Sertoli cells, they also have a large nucleus.
With regards to functions, Sertoli cells play an important role in the creation of the blood-testis barrier as well as providing nourishment for spermatozoa. Here, as already mentioned, cytoplasmic extrusions extend from the Sertoli cells and wrap around the germ cells. This increases the surface area of these cells and promotes the transfer of nutrients required to nourish germ cells.
In addition, Sertoli cells also secrete a number of substances including activins and inhibins, anti-Müllerian hormone, as well as androgen-binding proteins. These substances are involved in the development of the reproductive system.
On the other hand, Sertoli cells are primarily involved in the production, as well as influencing the effects, of testosterone. As such, they are heavily involved in spermatogenesis (production of spermatozoa from germ cells within the seminiferous tubules).
While the two types of cells have some differences (with regards to morphology and where they are located etc) they have been shown to interact/communicate. In adults, studies have shown that Sertoli cells play an important role in the maintenance of the Leydig cell population.
In an experiment where Sertoli cells were removed, 70 percent of the adult Leydig cells were lost leading to the conclusion that Leydig cells are dependent on Sertoli cells. On the other hand, Sertoli cell functions have also been shown to be dependent on the functions of Leydig cells.
The following is a diagrammatic representation of Sertoli cells and Leydig cells localization:
Return to learning about Sperm Cells
Return from Leydig Cells to MicroscopeMaster home
References
Cornelia Schulze. (1984). Sertoli Cells and Leydig Cells in Man.
Hisakazu Hayashi and R. G. Harrison. (1971). The Development of the Interstitial Tissue
of the Human Testis.
I Huhtaniemi and L J Pelliniemi. (1992). Fetal Leydig cells: cellular origin, morphology, life span, and special functional features.
Svechnikov K, Landreh L, Weisser J, Izzo G, Colón E, Svechnikova I, and Söder O. (2010). Origin, Development and Regulation of Human Leydig Cells.
Yuichi Shima. (2019). Development of fetal and adult Leydig cells.
Links
https://www.histology.leeds.ac.uk/male/sertoli_cells.php
https://www.sciencedirect.com/topics/neuroscience/leydig-cell
Find out how to advertise on MicroscopeMaster!