Flow Cytometry
Protocol, Principle, Basics, Applications and Results
Definition: What is Flow Cytometry?
Flow cytometry is one of the most popular laser-based technologies used to study a variety of cell (and particles) characteristics. As a laser-based technology, flow cytometry is largely dependent on the light-scattering properties of cells and particles that make it possible to analyze such characteristics as the size of cells, DNA content within a cell, as well as cell granularity among others.
One of the biggest advantages of this technique is that cells/particles are analyzed one at a time as they pass through the fluid stream. This makes it possible to analyze a variety of cell/particle characteristics. In some cases, staining (using dyes) or labeling the cells/particles (using fluorochrome-linked antibodies) is necessary for better analysis.
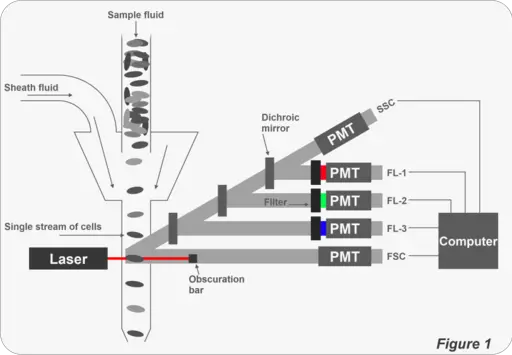
In general, a flow cytometer consists of the following core systems:
- Fluidics
- Optics
- Electronics
Overview
As mentioned above, a flow cytometer consists of three core systems. To analyze given characteristics of cells/particles, a sample fluid is first injected into the fluidics system in order to be transported through a narrow channel. This requires the use of sheath fluid that carries the cells/particles.
The sample fluid carries the cells/particles into a laser intercept where cells are analyzed one at a time as they fluoresce at specific wavelengths.
The light emitted by the cells then passes through a set of filters before reaching fluorescent detectors that measure their intensity: Here, detectors measure the intensity of fluorescence signals emitted by the cells/particles. The lasers used in flow cytometry are available at different wavelengths with varying levels of power.
Some of the lasers used today include:
- Argin lasers
- UV lasers
- Krypton lasers
- Helium-neon lasers
- Helium-cadmium lasers
Fluid Flow: Laminar Flow VS Turbulent Boundary Flow
In the flow chamber of a flow cytometer, two principles of fluid flow are often used.
These include:
Laminar Flow
In the laminar flow, cells/particles follow a well-defined path once they enter a small-bore tube. Here, the fluid front velocity at the inlet is the same at the walls as well as the center of the channel. However, as the fluid moves down the channel, viscous drag causes the outer layers of the liquid to slow down (liquid in contact with the walls of the channel).
As a result of this drag, the fluid front changes into a parabola. This is due to the fact that the center of the flow channel has the highest velocity while the outwards (parts in contact with the wall) have very little to no velocity.
The particles/cells are drawn to the center (area with the highest velocity) through hydrodynamic focusing. As the fluid runs down the channel, the parabola becomes increasingly stable which allows the cells to be analyzed one at a time.
* In a laminar flow, it's common for cells to appear elongated due to the hydrodynamic force.
* The inlet length, which is the distance between the inlet and the point at which a stable velocity parabola is formed, is about 50 times the diameter of the channel.
Turbulent Boundary Flow
In a turbulent boundary flow, a disordered flow pattern is evident compared to the defined path observed in laminar flow. This is because the rapid injection of the sample into the chamber, and thus the high speed of entry as well as back pressure resulting from the exit orifice contributes to sample turbulence that ultimately produces a viscous boundary at the interface with the sheath.
At this boundary, the drag generated produces a velocity parabola that draws particles at the center (this is because the center has the highest velocity).
Hydrodynamic Focusing
In flow cytometry, hydrodynamic focusing is the process aimed at solving hyperbolic velocity that causes cells/particles to move in a seemingly random manner (unfocused). Typically, this (random movement) occurs before the sample enters the flow chamber.
Here, cells/particles in the fluid suspension (sheath fluid) move in a random fashion and thus appear to be disorganized. This hyperbolic distribution is, for the most part, the result of some of the cells moving faster or slower than normal. This prevents the cells from moving in a single file line (in a straight path). In this scenario, it would prove difficult to analyze the cells/particles one at a time.
By using hydrodynamic focusing, the fluid forces the cells/particles to not only travel at the same speed, but also as single cells. Here, the fluid forces the sample into a smaller core (a channel with a smaller diameter) at the same speed into what is known as a hydrodynamic focusing region that narrows the channel thus focusing the sample in a smaller core stream. In the core stream, the layers move parallel to each other without mixing.
In flow cytometry, the pressure of the sheath fluid (which is basically a saline liquid) determines the rate at which cell/particle analysis occurs. However, it is important to maintain a low differential pressure for better analysis.
By increasing the pressure (differential pressure between the sample and sheath fluid), the event rate is increased which means that it becomes easier to obtain data faster. However, doing so presents a problem given that high-pressure results in the widening of the core stream which in turn allows more cells or particles to pass through.
As more cells/particles pass through the flow cell/flow chamber, then two or more cells pass through the interrogation point at the same time and thus two or more cells are illuminated at the same time which can produce undesired results.
By using low differential pressure, the cells/particles get to pass through the interrogation point one at a time because the core stream does not widen. Here, then, it becomes possible to analyze the cells one at a time and thus determine given characteristics of the sample.
* High differential pressure is often used in cases where researchers are not overly concerned about coincident events.
Acoustic-assisted Hydrodynamic Focusing
Acoustic-assisted hydrodynamic focusing is a relatively new development in flow cytometry aimed at mitigating the impacts of high pressure. As already mentioned, increasing fluid pressure causes the pore stream to widen thus allowing more cells/particles to pass through the interrogation at the same time. To mitigate this problem, then acoustic-assisted hydrodynamic focusing can be used.
Here, in addition to the hydrodynamic force, a device producing sound waves is also used for the purposes of aligning the cells so that they remained confined to a narrow region when pressure is increased.
Using this approach, researchers are able to use high pressure as the cells pass through the interrogation point one at a time. This, then, makes it possible to collect data faster without the disadvantages normally associated with high pressure.
Currently, two systems are used to produce the pressure used to move the sample.
These include:
· Differential pressure systems - Involves the use of a variable regulator to apply pressure to the sample tube
· Peristaltic pump systems - More precise than the differential pressure systems and are often used to generate absolute cell counts
Optic System
Essentially, flow cytometry entails the interrogation of cells by a laser. Fluorescence is then collected to analyze the characteristics of the sample. Therefore, optics plays a central role in the process. This optic system consists of a number of components that are appropriately arranged to generate the desired signals.
These include:
· Lasers - The lasers used in flow cytometry illuminate the sample to generate fluorescence signals from the sample. Such lasers as Argin lasers and UV lasers etc can be used.
* LASER stands for light amplification by stimulated emission of radiation
· Lenses - Lenses used in this technique serve to focus and collect light as well as fluorescence signals and directs them to the detector
· Mirrors - Used to direct the path of light in the system
· Filter - As is the case with a number of microscopes, filters are used to filter out the light before it reaches the detectors
For the laser light to illuminate the sample, the two have to interact. This interaction occurs at the interaction point as the cells pass through the channel in a single file line.
Laser is typically used because it is coherent and monochromatic. As a coherent source of light, lasers tend to be synchronized with an identical wave frequency. In addition, they tend to be energetic as compared to other light sources which allow illumination at a specific wavelength. Depending on the objectives/aim, one or several lines/beams of the laser can be used.
Laser Arrangement
Depending on the individual, different types of lasers can be used to illuminate the sample. Apart from the different types of lasers, users also have options when it comes to laser arrangements in flow cytometry.
In some cases, both options/arrangements can be used on the same instruments. In the first laser arrangement known as parallel arrangement, lasers are spatially separated from each other so that there is some distance between them.
In this arrangement, cells passing through the tube channel are exposed to one excitation source at a time. As a result, they are illuminated twice (or several times depending on the number of the light source) and thus emit light (fluorescence light) more than once.
In the second type of laser arrangement, the lasers are arranged in a manner that allows light to share the same optical pathway. In this case, the lasers are not separated which means that cells are illuminated and excited by several lasers at the same time.
Mirrors and Filters
In flow cytometry, a set of mirrors and filters are used for several purposes. For instance, the mirrors (dichroic mirrors) are types of filters used to reflect and thus direct light to the desired destination to not only illuminate the sample, but also ensure that emitted light (by the sample cells/particles) are detected by the detectors.
Here, the mirrors used may either be longpass filters (LP) or shortpass filters (SP) with mirror coating.
* Whereas LP filters (LP 500 filter) allow light above 500nm to pass through, SP filters (SP 500 filters) are designed to only allow light below 500nm to pass through.
* Mirrors used in flow cytometry are also referred to as beam splitters given that they are used to direct given wavelengths of light in the desired direction.
* Apart from mirrors, prisms may also be used to reflect light in flow cytometry.
Therefore, while mirrors are used to reflect and direct light in flow cytometry, they also serve to only allow a given wavelength of light to pass through.
In flow cytometry, filters are specifically used for the purposes of enhancing signals-to-noise ratio as well as reducing the crosstalk between fluorophores. For instance, filters are used for the purposes of isolating emission signals that are relatively weak as well as blocking neighboring fluorophores from passing through.
As these filters are typically used to filter the light emitted by the sample, they are known as emission filters. However, in cases where arc lamps are used to illuminate the sample, filters are also used to filter out undesired wavelengths and thus only allow specific wavelengths.
By using lasers as light sources, filtration is unnecessary given that the light produced is monochromatic.
* The wavelength of the light allowed to pass through the filter and reach the detector is close to the emission peak emitted by the sample of fluorescent dye used.
Apart from shortpass and longpass filters, filters known as bandpass filters (BP) are also used in flow cytometry. A good example of this is the bandpass filter with the label 530/30. It is a type of interference filter characterized by dielectric layers that only allows a specific wavelength to pass through.
Detection and Measurement
Following illumination, the sample are excited and emit fluorescence light that is then captured/collected by the detectors. Depending on the signal strength, a number of detectors may be used.
Two of the most common detectors used today include:
· Photodiodes - (PD) tend to be inexpensive and are often used to measure brighter signals
· Photomultiplier tubes (PMT) - As compared to photodiodes, photomultiplier tubes are more sensitive to lower signal levels. Because they can amplify energy from photons, they are suitable for measuring dimmer signals
Once the light signals reach and strike the detectors, they are converted into a number of electrons that have to be multiplied at the detector in order to produce an electric current. This current is then converted into voltage pulse (0 to 10 volts) at the amplifier. The pulse is then quantified in the electronic system of the instrument to measure signal intensity.
Fluorescence
When some cells or particles in the sample are illuminated (excited by the laser light) they emit light that is of a different wavelength to the illuminating light. This is known as fluorescence.
Because of the capacity of some cells/particles to absorb and emit light, it has become possible to use a number of instruments (e.g. microscopes and flow cytometer etc) to visualize and study a variety of properties without much difficulty.
While the ability of some cells/particles to fluoresce can be the basis of visualization and analysis, the natural level of fluorescence of cells can prove problematic in flow cytometry. This is largely due to autofluorescence caused by the presence of such cellular components as elastin, lysosomes, and mitochondria among others.
Given that autofluorescence is often detected at shorter light wavelength, the increased signal to noise ratio can negatively affect results.
* Less autofluorescence interference is caused by fluorophores that emit wavelengths above 600nm.
Fluorophores
Today, there are many types of fluorophores that can be used in flow cytometry.
Some of the most common of these include:
· Single dyes - Includes such dyes as PE and FITC
· Tandem Dyes - Dyes consisting of the primary and secondary fluorophores. Here, a small fluorophore is typically coupled (covalently) to another fluorophore
· Fluorescent proteins - These include such fluorophores as green fluorescent protein (GFP) and yellow fluorescent proteins
· Qdot antibody conjugates - Qdot antibody conjugates are excited by a wavelength of between 405 and 488 nm. Unlike tandem conjugates, these probes do not degrade over time and therefore have the advantage of greater reproducibility
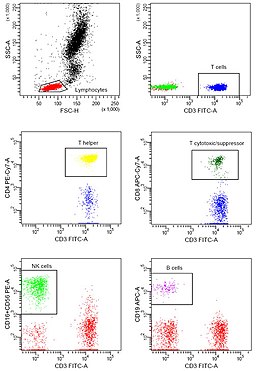
Application of Flow Cytometry
Today, flow cytometry has many clinical and research applications making it one of the most popular laser-based technologies in these fields.
In many laboratories, for instance, the technique has become a routine clinical method used for DNA analysis, immunophenotyping, as well as evaluating red blood cells and platelets.
Some of the other applications of flow cytometry include:
- Cell sorting
- Analyze cell cycle and apoptosis
- Assay of cell proliferation
Sample Preparation
Sample preparation is important in order to meet the basic requirements for cell/particle analysis. Regardless of the type of sample, it's important that, in the case of a biological specimen, cells are in single-cell suspension in order to prevent clogging caused by clumps.
Whereas peripheral blood mononuclear cells meet this requirement, cells in solid organs have to be prepared in order to use them in single-cell suspension.
Some of the other basic steps of sample preparation include:
- Removal of any unwanted contaminants
- Sample filtration to remove any clumps
- Avoiding excessive centrifugation to avoid pelleting the cells
- Sample enrichment in cases where the cells to be analyzed are rare (e.g. some white blood cells)
- Defrosting frozen cells and treating sensitive cells gently in order to avoid damage that can affect the quality of results obtained
Return to Blood Microscopy and Blood Smear technique
Return from Flow Cytometry to MicrsocopeMaster home
References
Antony C. Bakke. (2001). The Principles of Flow Cytometry. Department of
Deborah Michel. (2014). Basics of a Flow Cytometer. Pathology, Oregon Health Sciences University, Portland, OR.
James V. Watson. (1991). Introduction to Flow Cytometry.
M. G. Ormerod. (2000). Flow Cytometry: A Practical Approach.
Roger S. Riley and Michael Idowu. (2003). Principles and Applications
of Flow Cytometry.
Links
https://bitesizebio.com/30453/flow-cytometry-fluorophores/
Find out how to advertise on MicroscopeMaster!