Genus Cyclospora
Definition, Characteristics and Microscopy Observations
Definition
Formerly referred to as coccidia-like bodies/cyanobacterium-like bodies, the genus Cyclospora consists of unicellular coccidian parasites responsible for the intestinal infection known as cyclosporiasis. Although there is no conclusive evidence that the infection (cyclosporiasis) can be spread from humans to animals, Cyclospora-like oocysts have been identified in baboons and chimpanzees as well as cattle.
In human beings, the infection (caused by C. cayetanensis) is characterized by watery diarrhea, mild nausea, and abdominal cramping among other symptoms. In the acute phase, it has also been shown to cause general fatigue and anorexia. Taxonomically, Cyclospora is related to a number of other microorganisms including cystoisospora and Toxoplasma gondii.
Apart from C. cayetanensis, some of the other species belonging to the genus include:
- C. schneideri
- C. glomericola
- C. caryolytica
- C. niniae
- C. talpae
* While Cyclospora is particularly common in tropical and subtropical areas, it is distributed globally and thus found in both developed and developing countries.
General Classification
- Phylum: Apicomplexa
- Class: Coccidia
- Order: Eucoccidiorida
- Family: Eimeriidae
- Genus: Cyclospora
Characteristics of Cyclospora
Life Cycle
As already mentioned, human beings are the only hosts for Cyclospora cayetanensis. Being an obligate intracellular parasite, C. cayetanensis is therefore dependent on human beings (a single host) for their life cycle (both sexual and asexual stages). Here, the infection starts with the ingestion of sporulated oocysts in the environment.
According to studies, the transmission of oocysts may occur through the ingestion of contaminated water, food, or soil. However, this takes place after a given period of time (one to two weeks) following fecal excretion allowing the oocysts time to sporulate and consequently become infectious.
Apart from the three modes of transmission (which are often a result of poor hygiene), animal transmission has also been shown to cause human infection. For instance, in a number of studies conducted in developing regions (E.g. Peru, Nepal, Egypt, and Guatemala), contact with domestic animals was shown to result in infections in a number of cases.
* While contact with domestic animals has been shown to increase the risk of infection, there is no evidence of transmission of the parasite from human beings to animals.
* In the environment, sporulation of Clyclospora requires temperatures of between 22 and 32 degrees Celsius.
Following the ingestion (by susceptible hosts), the sporulated oocysts excyst in the gut where they release sporozoites. Once released, the sporozoites invade epithelial cells of jejunum and duodenum where they transform into trophozoites. Here, asexual multiplication (merogony) of the trophozoites results in the production of two types of schizonts (Type I and Type II schizonts).
Whereas Type I schizonts contain between 8 and 12 merozoites, Type II schizonts contain about 4 merozoites. Type II merozoites then differentiate into sexual stages (gametocytes) with the microgametocyte fertilizing the macrogametocyte to form the zygote. Once the zygotes are formed, they mature to form oocysts.
* The oocyst excreted into the environment is unsporulated and therefore has to undergo sporulation in the environment (under ideal environmental conditions) in order to be infective.
* Once they are exposed to air, about 40 percent of the oocysts sporulate and become infectious.
Morphology
In the environment (water, soil, etc) unsporulated oocysts are spheroidal in shape, measuring between 8 and 10um in diameter. This wall of the oocyst is thin and colorless with a bilayered structure.
Sporocysts, on the other hand, are characterized by an ovoidal shape and measure about 4× 6 µm. Unlike the oocysts which have a polar body and oocyst residuum, sporocysts stieda and substieda bodies as well as a characteristic large residuum.
* Each sporulated oocyst carries two sporocysts that contain two sporozoites each.
The sporozoites released during excystation are elongated in shape and may measure about 1 × 9 µm and lack refractile bodies. Meroziotes, on the other hand, vary in size with Type I merozoites measuring between 2 and 4um long while type II merozoites measure between 12 and 15um in length.
Mechanism of Pathogenesis
Like a number of other unicellular organisms that cause gastroenteritis, Cyclospora (C. cayentanensis) primarily affects the small intestine, and particularly the jejunum. Here, both the sexual and asexual forms of the parasite have been shown to invade the epithelial cells (enterocytes) where they undergo further multiplication and maturation to produce oocysts. As a result, the parasite ends up interfering with the normal absorption process.
Here, however, it's worth noting that even among those with a poor immune system, the number of oocysts released into the environment through the feces is relatively small.
Regardless, genetic diversity among some of the genetic sequences has led researchers to believe in the possibility of polyparasitism (where the infection is as caused by different strains of the parasite). Apart from enterocyte dysfunction caused by parasitic reproduction within the enterocytes, researchers also suspect C. cayentanensis pathogenesis to be the result of toxins released by the parasite.
* Apart from epithelial cells of the small intestine, oocysts of the parasite have also been detected in a number of nongastrointestinal samples. For instance, in two reports, oocysts of the parasite were detected in the sputa of HIV patients with a history of pulmonary tuberculosis. Based on these findings, it is suspected that Cyclospora might be an opportunistic pathogen.
Histopathology
Presence of Cyclospora has been associated with injury to the small intestine (jejunum in this case).
Some of the signs that may be detected through histological studies include:
- Erthema of the distal duodenum
- Focal vacuolization of the villi
- A loss of brush border
- Alteration of cell morphology - From columnar to cuboidal
- Villous atrophy
- Crypt hyperplasia
Risk Factors and Clinical Manifestations
Based on a number of epidemiological studies, children and those with a compromised immune system (E.g. HIV Patients) are at a higher risk. Whereas children have been shown to be five times more likely to be infected (and manifest signs of cyclosporiasis), higher rates of infection have been detected among HIV/AIDs patients.
Rates of infection have been shown to increase during warmer months (seasons). However, for the most part, the rates of infection in any region can be significantly reduced through good hygiene.
Although infection with cyclospora may remain asymptomatic (or mild) in many cases (especially in endemic areas), some of the symptoms among those in the risk group (children, HIV/AIDs patients, travelers, elderly) may include an onset of watery diarrhea (prolonged and intermittent), nausea, fever and abdominal cramps.
For a majority of immunocompetent patients, the symptoms are self-limiting and thus resolve spontaneously within a few days. However, for some of the patients, the symptoms persist, resulting in fatigue and loss of appetite. Along with bouts of diarrhea, some of the patients ultimately develop anorexia.
Adaptations of Cyclospora
In the environment, Cyclospora has been shown to be highly resistant to disinfectants used in water and food processing. This has been attributed to the ability of the parasite to strongly bind to such fresh produce as raspberries.
Using adhesins present on their surface, cyclospora is able to strongly bind to the fine hair-like projections on the topography on the fruit which allows the parasite retention on the fruit. While the oocysts of Giardia and Cryptosporidium are also capable of binding to given fresh produce, the stickiness is weaker compared to Cyclospora oocysts. In a number of countries, this adaptation has continually contributed to several outbreaks annually.
Microscopy
Currently, no serological assays (e.g. immunoassays and enzyme immunoassays) are commercially available for diagnosis purposes. However, a number of microscopic techniques can be used to detect oocysts in clinical samples.
Acid-fast stain (Modified acid-fast staining)
Before any step is taken, it's important to keep in mind that fecal samples are potentially infectious and should therefore be handled with care (using a pair of gloves).
Moreover, chemicals used are harmful to the skin, eyes and lungs (if inhaled). For this reason, they should also be handled with care. Here, protective cloths (e.g. a face mask) should be used and containers remain tightly closed when not in use.
Requirements
- Sample (stool sample containing oocysts)
- Staining rack
- Clean microscope slides
- Compound light microscope
- Tap water
- Bunsen burner/flame
- Fuchsin base (or safranin stain)
- Methylene blue
- Decolorizer (e.g. 1% H2SO4)
Sample preparation
Using a clean/sterilized wire loop or splint, scoop a small amount of the fresh stool and create a thin smear on the microscope slide - Try and make the smear as thin as possible.
Allow the smear to air-dry.
Pass the slide over a flame (Bunsen burner) 2 or 3 times in order to fix the smear - This should be done with care so as to avoid overheating the sample.
* While using fresh samples is recommended, fecal samples to be inspected in the future can be preserved using a number of solutions including 10 percent formalin, sodium acetate, polyvinyl alcohol, or sodium acetate acid formalin (SAF). However, doing this has a major setback given that oocysts are no longer viable. As a result, sporulation cannot occur.
To maintain the viability of oocysts, saline can be used (in place of formalin).
Staining procedure
· Once the slide has cooled, place it on the staining rack and flood with fuchsin base for about 4 minutes
· Gently wash the slide with running water - This should be done gently (with smooth-running water)
· Using 1% H2SO4, decolorize the sample for about 4 seconds
· Counter-stain the sample using Methylene blue for about 30 seconds
· Again, gently wash the slide with smooth-running water
· All the slide to air dry
· Observe the slide under the light microscope starting with 400x followed by 1000x magnification (use oil immersion for 1000x magnification)
Observation
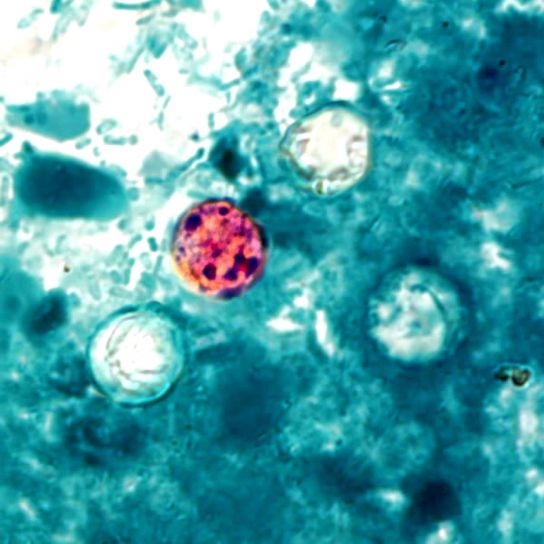
When viewed under the microscope, the spherical oocysts will appear pink or reddish in color.
* The background will appear blue in color due to the color of the secondary stain.
* Because Cyclospora oocysts stain poorly, they may appear as poorly stained/ghost cells along with well stained ones when viewed under the microscope.
Here, it's worth noting that like Cryptosporidium, the walls of Cyclospora oocysts contain acid-fast lipids are therefore resistant to decolorization. Unlike a number of other cells (e.g. some bacteria) they require heat fixing in order to allow for better penetration of the stain into the wall. This stain is retained during decolorization which causes the oocysts to appear pink in color when viewed under the microscope.
Cyclospora Autofluorescence
While modified acid fast staining is typically used to inspect samples for Cyclospora oocysts, fluorescent microscopy is proving to be one of the most useful methods for confirming their presence. This is because the method is more sensitive and yields better results (given that the oocysts exhibit autofluorescence under ultraviolet light) compared to permanently stained preparations.
* For this technique, no dyes are necessary. However, special stains may be used when the method is modified.
Wet Mount Procedure
Requirements
- Fluorescent microscope
- Saline
- Clean microscope glass slide
- Stool sample
Procedure
· Place a few drops (2 or 3 drops) of saline on a clean glass slide
· Using a clean splint, obtain a small portion of the specimen/sample and add it to the saline (mix the two)
· Place the preparation under the fluorescent microscope and check for autofluorescence of the oocysts at 200× magnification using about 330nm ultraviolet filter
Staining
To stain the preparation for fluorescence microscopy, about 10ul of Calcofluor White stain is added to the same amount of the sample on a clean glass slide. 0.05 percent of Evan's Blue Dye is then added to the preparation before examining the slide under UV fluorescence microscope at 405nm excitation wavelength.
* In fluorescence microscopy, probe 4, 6-diamidine-2-phenylindole (DAPI) may also be used to detect the presence of Cyclospora oocysts. Here, equal amounts of the sample and DAPI are placed on a clean glass slide for about 5 minutes with an equal quantity of Calcofluor white being added thereafter.
The slide is then allowed to air-dry before being viewed under the microscope (fluorescent microscope) using a 435-485 BA filter.
Observation
When viewed under a fluorescent microscope, the oocysts appear blue in color when ultraviolet excitation is set between 330-365nm and green in color as 450-490nm UV excitation with a darker background. This technique makes it easier to identify the oocysts given that they appear to glow in the dark.
Autofluorescence in oocysts of Cyclospora cayetanensis makes it easy to detect them in a sample. Essentially, autofluorescence refers to the ability of the oocysts (or other biological structures) to emit light when excited with UV light.
Like many other biological structures, the oocysts absorb and emit light when excited which in turn makes it possible to differentiate between them and other light in the field of view.
Prevention and Treatment of Cyclospora
(Cyclosporiasis)
Prevention of Cyclospora infection is an important step in that it helps protect the vulnerable group. Given that the parasite is transmitted through food, water, soil and domestic animals in some cases, then it follows that one of the best prevention strategies entails improving hygiene.
Basically, this involves such routines as washing hands with soap and water before handling/consuming fruits and vegetables as well as thoroughly washing fruits and vegetables.
Apart from cleaning hands and fresh produce, proper water treatment as well as the treatment of human sewage can help prevent Cyclospora infection (or at least significantly reduce the rates of infection) by affecting the life cycle of the parasite.
In third world countries where the infections are endemic, prevention can be achieved by properly boiling water (from rivers and other water bodies) as well as proper management of pit latrines. These strategies contribute to the general hygiene which in turn affects the life cycle of the parasite thus significantly reducing the rate of new infections.
For patients exhibiting the symptoms, TMP-SMX (Trimethoprim-sulfamethoxazole) is currently the antibiotic of choice. In one study where children were treated using the drug, they stopped excreting Cyclospora oocysts within a period of 3 days.
Apart from children, the antibiotic has also been shown to be effective for both immunocompetent and immunocompromised patients. For patients who are allergic to sulfonamides, nitazoxanide may be used to treat the infection.
Some of the other forms of antibiotic used to treat Cyclosporiasis include:
- Nitazoxanide
- Nalidixic acid
- Norfloxacin
- Azithromycin
Return to Unicellular Organisms
Return to Parasites under the Microscope
Return from Cyclospora to MicroscopeMaster home
References
Annunziata Giangaspero and Robin B Gasser. (2019). Human cyclosporiasis.
Jean-Marc Chavatte and Roland Jureen. (2016). Incidental Detection of Cyclospora cayetanensis during General Health Screening: A Case Study from Singapore
Leonor Chacín-Bonilla. (2017). Cyclospora cayetanensis. ResearchGate.
Ralph Lainson. (2005). The Genus Cyclospora (Apicomplexa: Eimeriidae), with a description of Cyclospora schneideri n.sp. in the snake Anilius scytale scytale (Aniliidae) from Amazonian Brazil - a review.
Sonia Almeria ,Hediye N. Cinar, and Jitender P. Dubey. (2019). Cyclospora cayetanensis and Cyclosporiasis: An Update. Microorganisms 2019, 7(9), 317.
Ynés R. Ortega and Roxana Sanchez. (2010). Update on Cyclospora cayetanensis, a Food-Borne and Waterborne Parasite. Clinical Microbiology Reviews.
Links
https://www.ncbi.nlm.nih.gov/pubmed/16021295
https://www.cdc.gov/parasites/cyclosporiasis/gen_info/faqs.html
https://web.stanford.edu/group/parasites/ParaSites2002/cyclospora/infection.html
Find out how to advertise on MicroscopeMaster!
![Global distribution of Cyclospora infections by Chacin-Bonilla, L. J.B. Rose B. Jiménez-Cisneros [CC BY-SA 3.0-IGO (https://creativecommons.org/licenses/by-sa/3.0-igo)] Global distribution of Cyclospora infections by Chacin-Bonilla, L. J.B. Rose B. Jiménez-Cisneros [CC BY-SA 3.0-IGO (https://creativecommons.org/licenses/by-sa/3.0-igo)]](https://www.microscopemaster.com/images/distributiongloballyofCyclospora_cayetanensis.png)
![Cyclospora Life Cycle, CDC [Public domain] Cyclospora Life Cycle, CDC [Public domain]](https://www.microscopemaster.com/images/Cyclospora_LifeCycle.png)