What are Alpha and Beta Tubulins?
Locations and Functions
Overview
Alpha and beta tubulins are globular proteins and exist included in five main forms/subunits as well as delta, gamma, and epsilon. As building blocks of various structures, tubulins belong to a group of proteins found in the cell cytoplasm in high amounts.
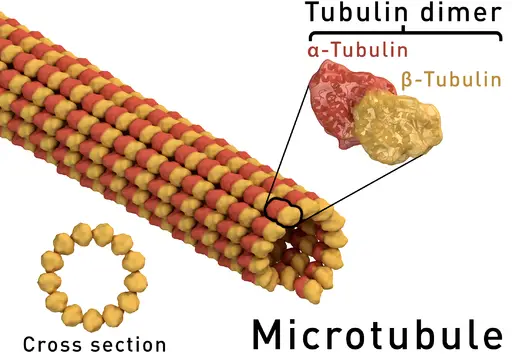
Alpha and beta tubulins, two of the most common and well-studied tubulins monomers are the building blocks of microtubule filaments and thus contribute to such biological processes as mitosis and intracellular transport among others.
General Structures of Alpha (α) and Beta (β) Tubulins
Essentially, alpha and beta tubulins have the same structure consisting of beta sheets and alpha helices. Based on molecular studies, alpha tubulin has been shown to consist of 13 percent of the alpha helices, 39 percent of beta-sheets as well as 48 percent of random coils.
On the other hand, beta tubulins consist of 13 percent alpha-helices, 42 percent beta-sheets and 45 percent of random coils. Here, each of the tubulin subunits is formed from two interacting beta-sheets that are then surrounded by the alpha-helices.
This composition produces compact monomers that consist of the following functional domains:
· Amino-terminal domain - Nucleotide-binding site: Also known as the N-terminal, this domain forms the Rossman fold and consists of five alpha helices and six beta-strands that are parallel to each other.
· Intermediate domain - The intermediate domain contains the taxol binding site and consists of three alpha sheets and beta-sheets.
· Carboxy-terminal domain - This domain contains the binding site for motor proteins. Also referred to as the C-terminal, the carboxy-terminal domain consists of alpha-helices.
While the two monomers (α and β) have a similar structure, differences can be identified in their amino acid sequence as encoded by different sets of genes. Whereas alpha-tubulin contains Asp-245 at their E-site, the N-site of beta-tubulin contains Lys-254.
On the other hand, alpha tubulins have an attached GTP (a purine nucleoside triphosphate) which is exchangeable with GDP in beta-tubulin.
* Alpha tubulins are encoded by TUBA genes while beta tubulins are encoded by UBB genes.
Microtubule Formation (Assembly of Alpha and Beta Tubulins)
In order to form microtubules, alpha and beta monomers join to form dimers that in turn form protofilaments. For each dimer (consisting of an alpha and beta tubulins) both the monomers are nucleotide-binding proteins, which drives assembly of the filament (microtubule).
Following assembly of the two monomers, the GTP of the alpha tubulin is buried within the protein and thus not exposed to the surrounding environment. For this reason, the GPT of the alpha tubulin in the dimer cannot be hydrolyzed.
In the beta subunit, however, GTP is exposed to the environment. For this reason, it's easily hydrolyzed. In the process, dimmers assemble to form a protofilament. Hydrolysis of the GTP provides the energy required for the assembly of more dimers onto the structure (protofilament).
The heterodimer orientation is polar in nature with the alpha tubulin subunit being the minus (-) end while the beta tubulin being the plus (+) end. As the dimers assemble to form the protofilament, addition of tubulins at the minus end is significantly slower compared to the plus end.
Protofilaments formed through tubulin addition are then arranged side by side to form a sheet that then folds to form a microtubule. Here, it takes about 13 protofilaments to form a single microtubule.
Due to the polarity of the dimers, the microtubule formed from protofilaments also has a plus and minus end. In this structure, dimers may continue being added at both ends. However, as already mentioned, very few dimers are added to the minus end as compared to the plus end.
As the heterodimers are added to the microtubule, GTPase activity of beta tubulin is activated. This contributes to locking the tubulin (beta tubulin) in the structure into a GDP-bound state. On the other hand, this tubulin forms a GTP cap that prevents the depolymerization of the microtubule given that it is exposed to solvent.
Some of the main characteristics of microtubules include:
- Resemble hollow tubes
- Length can be about 1000 times their width
- Are randomly located in plant cells
- Appear to originate at the centrosome in animal cells
- Have a diameter of about 25 nanometers
- Exist for a very short period of time (about 10 minutes) because they are highly unstable (dynamic unstable)
- Assemble quickly depending on the needs of a cell
Importance of Alpha and Beta Tubulins
As mentioned, the alpha and beta tubulins are building blocks of microtubules.
This produces a strong cylindrical structure which serves the following functions:
Cell Cytoskeleton
Alpha and beta tubulins are the building blocks of microtubules, one of the three components of the cell cytoskeleton. As compared to the other proteins (microfilaments and intermediate filaments) that make up the cell cytoskeleton, microtubules are larger (in diameter).
Together, these components make up the infrastructure of a cell (influence cell shape and provide support as well as contributing to movement within the cell)
Cell Division
Microtubules give rise to spindle fibers (mitotic spindle) involved in separating chromosomes during anaphase. This allows chromosomes to be separated and pulled to the opposite poles of the cell. In the process, they ensure that each daughter cell contains the same number of chromosomes.
Cilia and Flagellum
Cilia and flagellum structures (e.g. in male sperm cells, female fallopian tubes, and prokaryotic cells, etc) contain microtubules. In flagellum structure, for instance, doublet microtubules surround a central pair of microtubules in a 9+2 arrangement.
In both cilia and flagellum, microtubules contribute to movement (e.g. of sperm cells and various unicellular organisms) and various functions of given body organs (e.g. movement of the egg along the fallopian tube)
Role of Tubulin in Cancer
While alpha and beta tubulins contribute to various functions in the body as well as in many unicellular organisms, their alterations have been associated with the development of cancer.
In particularly, alteration of tubulin isotype expression has been shown to be one of the main causes of cancers. For the most part, this has been attributed to the fact that TBAs can bind to beta tubulin resulting in toxic effects.
Apart from changes in the composition of tubulin isotype, some of the other factors that may contribute to cancer include:
- Post-translational modifications of tubulin
- Microtubule-associated proteins
Return to Eukaryotes main page
Return from Alpha and Beta Tubulins to MicroscopeMaster home
References
Amelia L. Parker1, Maria Kavallaris and Joshua A. McCarroll. (2014). Microtubules and their role in cellular stress in cancer.
Eva Nogales, Sharon G. Wolf & Kenneth H. Downing. (1998). Structure of the abtubulin dimer by electron crystallography.
Pavla Binarová and Jack Tuszynski. (2019). Tubulin: Structure, Functions and Roles in Disease.
Links
https://www.letstalkacademy.com/publication/read/formation-of-microtubules
https://www.cureffi.org/2013/03/10/cell-biology-06-the-cytoskeleton-part-ii-tubulin/
Find out how to advertise on MicroscopeMaster!